Protein aggregation needs to be addressed for the successful development and quality control of new pharmaceutical formulations and drugs. The presence and proportion of aggregates in such biopharmaceuticals can be directly related to the bioavailability, drug activity, and potential negative side effects like adverse autoimmune reactions.
Especially as the use of monoclonal antibodies as therapeutic agents is increasing, but potential negative immune responses could be triggered by the formation of aggregation of the antibody as a result of the formulation process involved1.
Classical Characterization Methods
The need for simultaneous separation of monomeric, oligomeric and aggregated antibody species makes the analytical characterization of such antibodies a challenging task.
Size exclusion chromatography (SEC) and analytical ultra centrifugation (AUC) are the traditional methods for this purpose, but these approaches have their own limitations when applied to the characterization of antibody aggregates.
A high-resolution size separation, quantification, and characterization of antibody aggregates can be achieved with AUC, but a high capital and operation cost is involved, limiting reproducibility. The need for skilled operators restricts the adoption of AUC as a standard analytical tool and AUC does not allow for the collection of the size-classified fractions for subsequent analysis or activity determination.
Until now, SEC has been the most widely used separation method for antibody aggregate characterization in bio-pharmaceuticals. The limitations of SEC are largely associated with the stationary phase used within the columns.
Inaccurate results are obtained at any time because of shear effects modifying the samples and affecting their bioactivity, low recovery due to protein adsorption onto the column materials, and filtering of aggregates through the columns matrix.
Moreover, the organic modifier and eluents needed by the special column packing can modify the antibodies and alter the ratio of aggregates detected at the column outlet.
The size exclusion limit of the column is another limitation, restricting the upper molar mass and particle size range of SEC. Consequently, smaller particle sizes and lower amounts of aggregates tend to be observed in SEC compared to AUC or FFF.
AF4 - The Modern Alternative
Asymmetric flow field-flow fractionation (AF4) is an advanced separation technique that can address the problems connected to AUC and SEC.
This innovative method is the modern alternative to meet all these characterization needs. In AF4, an unpacked separation channel is used in place of a packed column. The absence of a stationary phase offers the following benefits:
- No shear forces
- Fast separation times
- Matrix interaction-free sample separation
- Great flexibility with regard to the selection of solvent
Another advantage of AF4 is its wide separation range: 1kDa / 1nm up to several MDa / 10µm. This capability enables AF4 to provide a complete picture of the aggregation phenomena, such as monomer, insoluble and soluble aggregates, as well as the subvisible particle fraction.
Advanced AF4 Detection by MALS and DLS
AF4 is an elution-based technology and can be easily integrated into advanced on-line detectors for obtaining multi-dimensional data from a single injection. The standard detector used for the identification and quantification of aggregates is UV.
Dynamic light scattering (Malvern Zetasizer Nano DLS) and multi-angle light scattering (Postnova PN3621 MALS) are sophisticated detectors capable of determining particle size and absolute molar mass, which can be used for subsequent analysis of separated aggregates.
With all these advantages, AF4-MALS has recently been recommended by the FDA for any analysis of antibody formulations and for obtaining supporting data with any new application in the future2.
Comparison of AF4, AUC and SEC Data
Figure 1 shows the results of the characterization of a monoclonal therapeutic antibody sample comprising of aggregates, demonstrating the performance of AF4 technology.
A Postnova AF2000 Focus Asymmetrical Flow FFF coupled to PN3211 UV Detection was used to perform this analysis. The same sample was also analyzed by AUC and SEC. The amount of aggregates determined by all the three methods is presented in Table 1, highlighting the performance capabilities of AF4 technology.
Table 1. Amount of Aggregates obtained from AF4, AUC and SEC
|
|
AF4
|
AUC
|
SEC
|
|
Amount Aggregates %
|
28.00
|
24.00
|
1.00
|
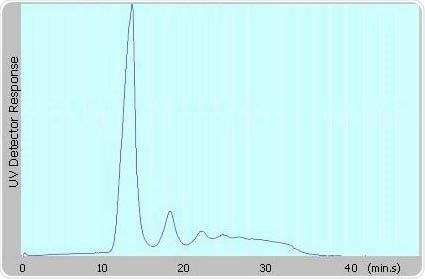
Figure 1. Fractogram of antibody obtained from AF4-UV @ 280nm
From the results, the amount of aggregates measured by AUC (24%) and AF4 (28%) agree well with each other, but clearly differ with the value (1%) obtained from SEC measurements.
These results suggest that most of the aggregates could have already been degraded and filtered out by the column material or adsorbed onto the column.
Conclusion
Although Asymmetrical Flow FFF provides information comparable to SEC, the absence of packing material offers a number of advantages, particularly no size exclusion limit and low shear conditions eliminating degradation of aggregates.
Ease of operation, compatibility for autosampler use, and amenability for the physical fractionation of separated sample material are the key advantages of the AF4 system over AUC. The results presented in this article highlight the ease of operation of AF4 compared to AUC and its ability to provide highly accurate results for aggregates compared to SEC.
Acknowledgements
Produced from materials originally authored by Dr. Soheyl Tadjiki, Thorsten klein and Evelin Moldenhauer from Postnova.
References and Further Reading
- Guidance for Industry on Immunogenicity Assessment for Therapeutic Protein Products, Food and Drug Administration (FDA), April 2014.
- Draft Guidance for Industry on Immunogenicity-Related Considerations for the Approval of Low Molecular Weight Heparin for NDAs and ANDAs, Food and Drug Administration (FDA), August 2014.
About Postnova Analytics
 Postnova Analytics' mission is to solve analytical problems in biotechnology, polymer and particle science.
Postnova Analytics' mission is to solve analytical problems in biotechnology, polymer and particle science.
Advanced Analytical Products
- Postnova always and only offers high quality products
- Postnova offers the newest and most innovative technologies available
- Postnova only chooses leading manufacturers as supply partner
Powerful Product Portfolio
- Postnova has formed a complete product portfolio (systems, supplies, services)
- Postnova has focused only Field-Flow Fractionation and Light Scattering
- Postnova offers complete solutions from one source with high expertise
Strong Customer Support
- Postnova has a highly qualified customer support team
- Postnova is always looking for long term partnerships with our customers
- Postnova provides the ultimate solution to make customers successful: Quality & Support
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.