A critical issue in the pharmaceutical and clinical field is the osmolality of solutions which must be regularly monitored. This includes infusion solutions as well as solutions meant for external use like eye drops; and to guarantee the physical well-being of human beings the rinsing solutions should be isotonic. In this analysis, the KNAUER K-7400S Semi-Micro Osmometer was used to determine the osmolality of commercially available pharmaceutical solutions.
The osmolality is traditionally consulted as an assessment value to ensure the quality of solutions used for medical or pharmaceutical purposes. As a standard measure for the number of molecules solved in a liquid, the osmolality is typically given in mOsmol/kg. For instance, Ringer solution [1], 5% glucose and physiological salt solution (0.9% NaCl) are traditionally used for clinical applications.
These solutions need to be in the osmolality range of 290±10 mOsmol/kg to conform to human plasma [2]. Apart from these physiological infusions, higher concentrations of glucose solutions (10%, 15% and 20%) are also used in day-to-day clinical practice.
These are either used as a carbohydrate component in parenteral nutrition or used for treating hypoglycemic conditions [3]. All the afore-mentioned solutions were prepared and examined to assess their real osmolalities.
Results
In order to assess the results, the solutions were divided based on different fields of applications. The isotonic solutions are used for rinsing or infusion, while the glucose solutions with different concentrations are used for special treatments. Being an exception, the 5% glucose solution can possibly be used for both ambits.
Shown in Figure 1 is the measurement of the isotonic solutions with respect to the specified limit value for plasma. Further, the averaged osmolalities (n=10) for Ringer solution, 5% glucose and 0.9% NaCl were 284 mOsmol/kg, 287 mOsmol/kg, and 281 mOsmol/kg, respectively.
Figure 2 shows the quantified osmolalities of various glucose solutions with 20%, 15%, 10% and 5% concentrations. The measured averaged osmolality (n=10) added as much as 1299 mOsmol/kg for 20% glucose; 939 mOsmol/kg for 15% glucose; and 604 mOsmol/kg for 10% glucose.
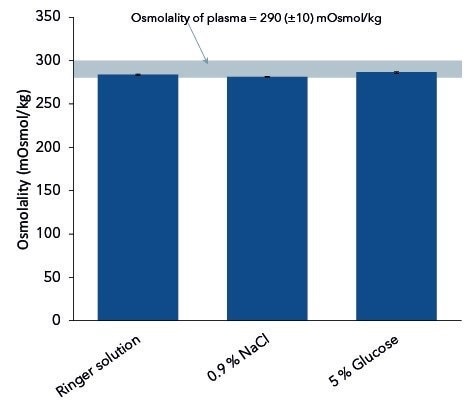
Figure 1. Measured osmolalities of pharmaceutical infusion solutions. Graph shows average values and standard deviations of 10 replicates.
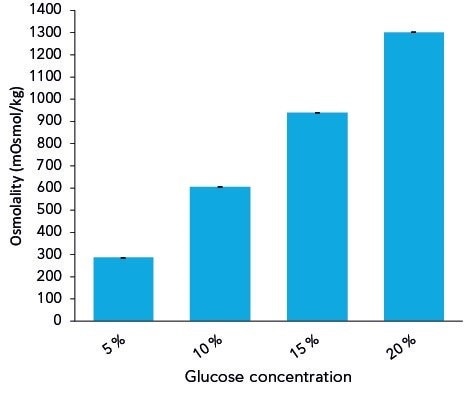
Figure 2. Measured osmolalities of different glucose solutions. Graph shows average values and standard deviations of 10 replicates.
Materials and method
The KNAUER K-7400S Semi-Micro Osmometer was used to make all measurements. Osmolality values of 2000, 850, 400 and 300 Osmol/kg were exhibited by the used calibration standards. The system parameters were then set to -8 °C for freeze and -16 °C for cooling limit, respectively.
Using an ultrasonic bath, all prepared solutions were degassed to remove carbon dioxide. Following this, 150 μL of these samples were transferred to a plastic sample tube.
Conclusion
For all isotonic solutions, the measured results were within the specified limit value in the osmolality range of 290±10 mOsmol/kg for human plasma. This is quite remarkable because a lot of pharmaceutical solutions manufacturers only give the hypothetical osmolalities of their products.
In addition, the hypothetical osmolality of physiological salt solution is given as 308 mOsmol/kg but as demonstrated in the study, the actual osmolality is apparently lower (281 mOsmol/kg).
In the case of relatively complex infusion solutions, it is strongly recommended to verify the actual osmolalities because the difference to the hypothetical value may be even higher. If no limit values of osmolality exist for glucose solutions of higher concentration, the measurements can be employed as a good example to demonstrate that osmolality comportment is not linear.

Additional results
Figure 3. Temperature-time-curve
Additional materials and methods
Table 1. Method parameters
|
Calibration 1
|
0 mOsmol/kg
|
300 mOsmol/kg
|
400 mOsmol/kg
|
|
Calibration 2
|
0 mOsmol/kg
|
300 mOsmol/kg
|
850 mOsmol/kg
|
|
Calibration 3
|
0 mOsmol/kg
|
850 mOsmol/kg
|
2000 mOsmol/kg
|
|
Sample volume
|
150 μL
|
|
|
|
Freeze
|
-8 °C
|
|
|
|
Cooling limit
|
-16 °C
|
|
|
Table 2. System configuration & data
|
Instrument
|
Description
|
Article No.
|
|
Osmometer
|
KNAUER K-7400S Semi-Micro Osmometer
|
A0006AC
|
|
Sample tubes
|
Approved plastic sample tubes, 500 pcs.
|
A0272
|
|
Software
|
EuroOsmo 7400
|
A3705
|
References
- http://flexikon.doccheck.com/de/Ringer-L%C3%B6sung
- https://www.gesundheit.gv.at/labor/laborwerte/niere-harn/osmolalitaet
- https://www.serag-wiessner.de/fileadmin/redakteur/
About KNAUER
 KNAUER is an owner-managed middle-sized company with more than 120 employees situated in Berlin, Germany. Since 1962, KNAUER has been producing and developing high performance liquid chromatography (HPLC) systems.
KNAUER is an owner-managed middle-sized company with more than 120 employees situated in Berlin, Germany. Since 1962, KNAUER has been producing and developing high performance liquid chromatography (HPLC) systems.
The HPLC technique is used to separate, identify and quantify substances in a mixture. It can be used for many applications, including the analysis of toxics in drinking water, the detection of drugs in blood as well as for the purification or quality inspection of pharmaceutical and chemical products.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.