The KNAUER K-7400S Semi-Micro Osmometer is specifically designed to determine the true freezing point of aqueous solutions. As a result, the osmolality of different types of samples like soft drinks and pharmaceutical solutions can be easily measured.
Theory of Osmolality
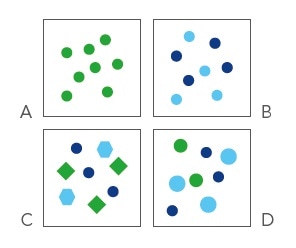
Osmolality is a standard measure of the concentration of particles in a solute. It is only based on their number, not on the nature of molecules.
Hence, the same osmolality is exhibited by a two molar solution of a non-dissociating molecule (A) just like the one molar solution of a fully dissociating salt containing two ions (B). Even when molecules differ in shape (C) and size (D), a solution will remain the same.
As a result, the same osmolality is exhibited by all solutions that contain the same number of osmotically active particles, irrespective of their chemical properties.
Freezing Point Osmometry
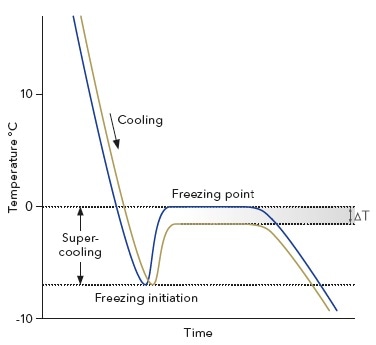
The K-7400S’s measurement principle is built on the colligative property of freezing point depression. For instance, the freezing point of the solution will decrease on adding a solute to a liquid. The depression is 1.858 K per 1 mole of ideally solved compound in one liter of water.
This effect depends solely on the number of particles in the liquid and not on the physical or chemical properties of the solutes. Due to this linear correlation, the osmolality of a sample can be determined by precisely measuring its freezing point.
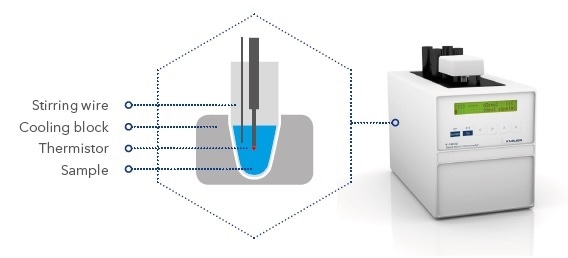
Schematic illustration of the measurement unit
Measuring Process
A microprocessor-controlled peltier element is used to cool the sample at the beginning of the measurement. During this procedure, the solution is super-cooled to below 0 °C, but remains in a liquid state. At a specific temperature, the freezing process is triggered by rotating the stirring wire.
When ice crystals are formed, thermal energy is released raising the sample’s temperature. After a brief gap, an equilibrium is said to be reached when melting and thawing of ice crystals are balanced and the temperature of the sample remains constant. This plateau represents the sample’s actual freezing point.
During the entire procedure, a high-precision thermistor is used to measure the solution’s temperature. A resolution of 1/1000 K allows accurate determination of the freezing point temperature and also enables the measurement of slight variations in osmolality of two samples.
About KNAUER
 KNAUER is an owner-managed middle-sized company with more than 120 employees situated in Berlin, Germany. Since 1962, KNAUER has been producing and developing high performance liquid chromatography (HPLC) systems.
KNAUER is an owner-managed middle-sized company with more than 120 employees situated in Berlin, Germany. Since 1962, KNAUER has been producing and developing high performance liquid chromatography (HPLC) systems.
The HPLC technique is used to separate, identify and quantify substances in a mixture. It can be used for many applications, including the analysis of toxics in drinking water, the detection of drugs in blood as well as for the purification or quality inspection of pharmaceutical and chemical products.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.