For the majority of laboratories over a range of industries, meeting regulatory compliance is a necessity. This task can be made much more achievable through the use of Internet of Things (IoT) sensors, in combination with data storage, retrieval and reporting abilities.
This article will identify ten ways in which employing IoT sensors can allow you to attain Good Laboratory Practices (GLP). Elemental Machines’ sensors and system have been developed for operation in regulated environments by meeting FDA regulations 21 CFR Part 11B Electronic Records, 21 CFR Part 58D GLP Equipment and 21 CFR Part 1271 Human Cells and Tissues.
Sensors produced by Elemental Machines are factory calibrated and can be calibrated to NIST-traceable standards.

About 21 CFR Part 11B Electronic Records
As part of an FDA 21 CFR 11 compliant process in food, drug, medical and science-based research environments, the Elemental Machines Platform gathers electronic records securely. For compliance with 21 CFR 11, it is necessary that a system meets particular controls to guarantee authenticity, integrity and confidentiality of electronic records.
The Elemental Machines Insights Platform:
Meets Electronic Record Authenticity Requirements:
It is a requirement of 21 CFR Part 11 that the system be precise and dependable, as well as have the capacity to identify invalid or altered records. The sensor data in Elemental Machines is encrypted in transit using HTTPS protocol, stored redundantly, and once recorded, cannot be modified by users.
All data is logged with a time-stamp, and data authenticity is confirmed upon ingestion, storage and there-after. There are no boundaries on time or quantity in regards to data stored by Elemental Machines, and all data is habitually backed up over distributed databases.
Meets Electronic Record Integrity Requirements:
It is a requirement of 21 CFR Part 11 that human readable copies of records can be produced without any impact on the original record.
Sensor data from Elemental Machines can be examined from a browser in a variety of formats, including historical graphs, time-stamped copies produced in general reports, and data manually exported in spreadsheet/text form, or programmatically exported using a secure API.
Whichever form is used, data is made available to authenticated users in a format that is both standard and readable.
Meets Confidentiality Requirements:
It is a requirement of 21 CFR Part 11 that access to the system is restricted to authorized users only.

In order to authenticate users to this standard or higher, Elemental Machines requires that as a minimum, a username and password are needed for access, while roles-based access can also be given to authorized users, limiting approved actions to pre-selected functions.
Logged data cannot be accessed by unauthorized users, and customer data is separated by accounts to make sure that users from one customer are unable to access the data of another.
Note: To ensure full compliance with 21 CFR Part 11, an organization’s complete system of data collection and recording must meet regulations. This system includes not just the use of Elemental Machines equipment, but also manual procedures, user training, and validation.
21 CFR Part 58D GLP for Lab Equipment
Elements & Dashboard features have been designed to allow for the reporting of temperature and environmental data for the customer’s study protocol over a range of methods, including dashboard view, logfile export, PDF report and API.
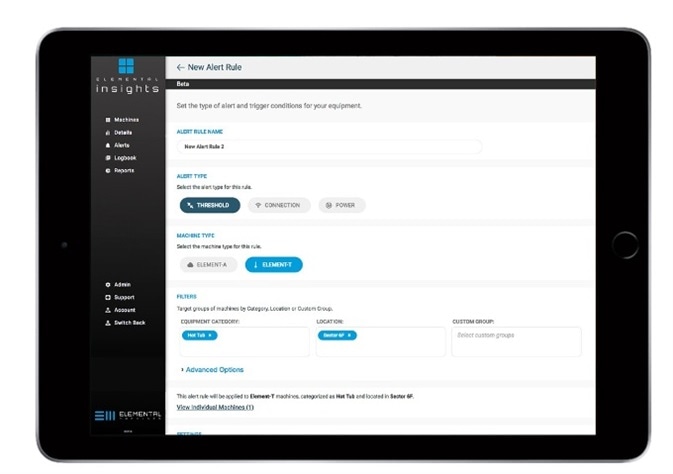
Wireless Elements Can Be Attached In a Variety of Ways
Wireless elements, such as the no-tool magnetic attachment or removable adhesive strips, can be attached in a number of ways, allowing access for inspection, cleaning and maintenance.
Elements Are Factory Calibrated
Factory calibrated elements are available with NIST-traceable calibration. They can also easily be calibration verified in-field.
Detailed Product Sheets
Detailed product sheets which describe inspection, cleaning, maintenance, testing, and calibration for sensor elements are available.
Element and Platform Operation and Trouble-Shooting Information
Information on how to use the product, as well as a bank of solutions to common issues, is accessible both online and through customer support.
Elemental Insights Dashboard
The Elemental Insights dashboard delivers dedicated, traceable fields for calibration date, installation date and other user operations, including documentation of unplanned repair and corrective actions. These can be exported into a customer’s QMS system via reports.
21 CFR Part 1271 Human Cells, Tissues and Cellular & Tissue-Based Products
The Elemental Machines sensors and platform can be employed to aid in compliance with FDA 21 CFR Part 1271.
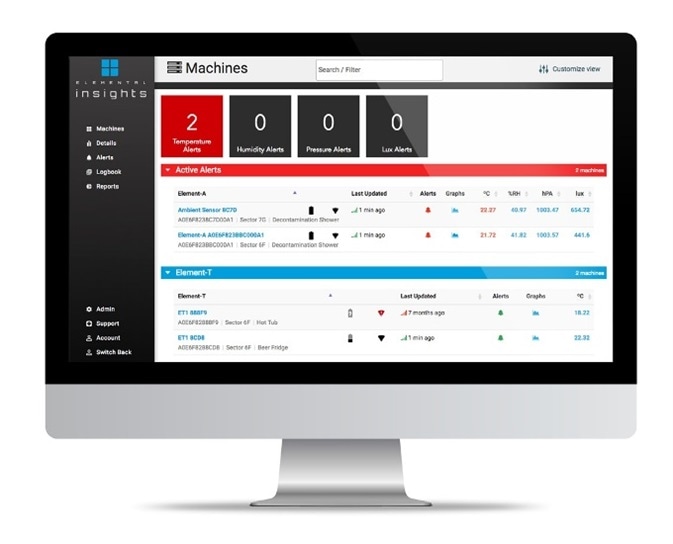
Meets Alerts and Monitoring Requirements
- Elemental Machines sensors and platform can be employed to constantly track, document, and maintain records of monitoring activities in freezers, refrigerators, cold rooms, and other storage areas
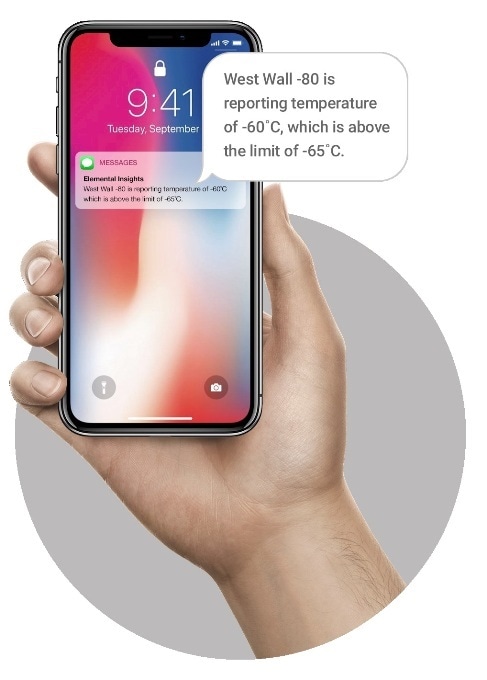
- It is possible to set pre-defined limits for alert thresholds and have the system proactively notify when these limits are exceeded, producing an audit trail and capturing related corrective actions
- Cloud-based storage delivers adequate readable access for periods beyond 10 years
- The Elemental Machines system saves and can reproduce electronic records as required through log download and reports
Meets System For User Generated Records Requirements
The Elemental Machines system offers annotation fields that enable users to document cleaning, disinfection, maintenance and inspection activities related to Elemental Machines sensors. This information can subsequently be arranged in the form of reports, ready to be entered into the customer’s QMS system.
About Elemental Machines
Elemental Machines is helping scientists improve experimental reproducibility and accelerate scientific discovery.
From early research and discovery to manufacturing, everyone in biology and chemistry-based industries knows that the physical environment can affect the entire product lifecycle. What they don’t know is exactly how, or the cumulative cost of not knowing -- higher R&D expenses, slower time to market for innovative products and life-saving therapies, yield loss during manufacturing, and more.
With a deep understanding of these issues, Elemental Machines is delivering unprecedented insight into complex processes, helping customers refine and accelerate their work across all phases of product innovation.
By gathering and synthesizing environmental data into actionable information, the Elemental Machines Sensory Network™ provides critical insights that improve transparency, repeatability and outcomes, and save customers time and money.
Track contextual variables (temperature, humidity, air pressure and light) in the research lab or monitor critical equipment performance (freezers, refrigerators, ovens, and incubators) for easy access to performance data, as well as alerts if readings are out of range.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.