A neurodegenerative disorder characterized by the aggregation of alpha synuclein into Lewy bodies in the brain is known as Parkinson’s Disease (PD). Alpha synuclein is a 14-kDa protein encoded by the SNCA gene.
Regarding the native state of alpha synuclein, there is conflicting evidence: it may be a folded tetramer, an unfolded monomer,1 or a dynamic equilibrium between these and other oligomers.2
These smaller forms aggregate into fibrils, protofibrils, and ultimate Lewy bodies in Parkinson’s Disease, which are thought to lead to neuronal dysfunction and death. There is also the possibility that the protofibrils and oligomers are the neurotoxic species3-6 while Lewy bodies are neuroprotective.7
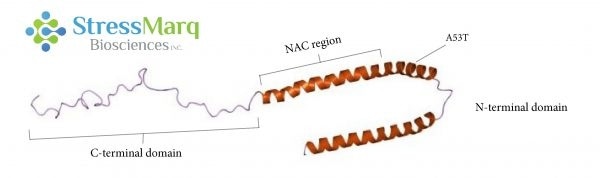
Alpha synuclein consists of three main regions: the C-terminal domain, the NAC region, and the N-terminal domain. The A53T mutation is found in the N-terminal domain. Image Credit: StressMarq Biosciences
What is the A53T Mutation?
A53T is a missense point mutation, this means that one amino acid is altered: the 53rd amino acid is changed from alanine to threonine. This mutation is a result of guanine being changed to adenine at position 209 of the SNCA gene (G209A).8
What is the Role of the A53T Mutation in Parkinson’s Disease?
First documented in families of Greek and Italian descent, the A53T mutation has been associated with autosomal dominant, early-onset PD.8 It has also been recorded in a Korean family.9 A53T mutation leads to an earlier age of onset and a shorter disease duration.8
Even though most cases of PD are not inherited, and are sporadic without involving the A53T mutation, understanding the role of A53T could help researchers better understand the mechanisms of alpha synuclein aggregation and create better therapies and models.
A53T Aggregation
A53T alpha synuclein fibrillizes in solution quicker than wild-type (WT) alpha synuclein.10 However, the mutation has a bigger influence on the rate of alpha synuclein protofibril formation than on the rate of fibril elongation.11
The fact that A30P and A53T mutations both promote protofibril formation and are both associated with early-onset PD, indicates that protofibrils contribute to the neurodegeneration seen in PD.12
Why does A53T Alpha Synuclein Fibrillize Faster than WT Alpha Synuclein?
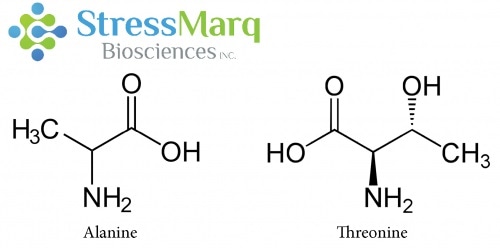
Alanine and Threonine have similar structures, but substituting Threonine for Alanine in alpha synuclein has significant effects on alpha synuclein fibrillization. Image Credit: StressMarq Biosciences
Why is there such a significant effect when the A53T mutation only involves a single changed amino acid, and threonine and alanine are structurally similar? A53T and other disease-causing mutations happen in the N-terminal part of the alpha synuclein protein.
Theoretical models suggest that the A53T mutation leads to long-range interactions between the N- or C-terminal regions and the NAC region of alpha synuclein to disappear, resulting in a growth in beta-sheet formation.13
NMR measurements suggest that the A53T mutation can extend and stabilize beta-sheet structures which are involved in fibrillization and oligomerization.14 Cryo-EM analysis showed that alpha synuclein fibrils are made up of two protofilaments twisted together into left-handed helices.16
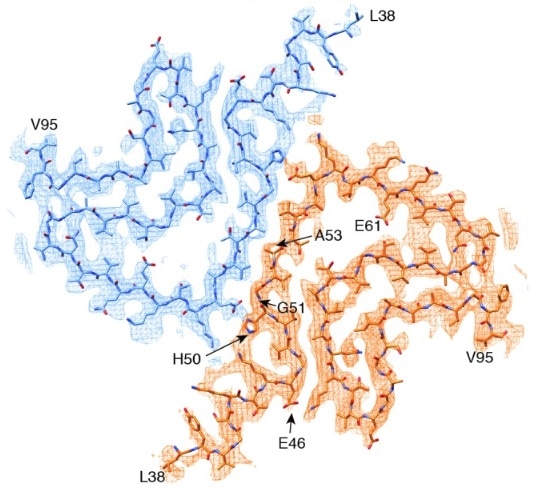
The cryo-EM-determined structure of alpha synuclein shows most disease-causing mutations to be located at the interface between two protofilaments.15. Image Credit: doi.org/10.7554/eLife.36402.001 © 2018, Guerrero-Ferreira et al.
Together with four of the five other early-onset PD mutations, the A53T mutation is found at the interphase between the two protofilaments.16 Introducing a hydrophilic residue like threonine disrupts this “steric-zipper interphase” and alters the structure of the fibrils.16
Effects of the A53T Mutation
Regardless of the mechanism of A53T alpha synuclein aggregation, it has devastating downstream effects. A53T alpha synuclein expression in mice results in paralysis, motor deficits and death.17
A53T alpha synuclein is more inclined to form annular and tubular protofibrils than WT alpha synuclein, and A53T alpha synuclein protofibrils bind tightly to phospholipid vesicles, resulting in their permeabilization.12
By enhancing nucleation of monomers, these lipid vesicles can induce fibril production.18 It is thought that the A53T mutation promotes fibrillization by either altering the structure of vesicle pores or by promoting the formation permeabilizing protofibrils over other species.19
Synapses
Synapses are components of neurons which are responsible for the transfer of information between neurons via synaptic vesicles.23 By depleting the synaptic vesicle recycling pool, both A53T and WT alpha synuclein suppress presynaptic transmission.24
A53T alpha synuclein expression also generates postsynaptic deficits.25 These postsynaptic deficits are due to the hyperphosphorylation of tau and its mislocalization to dendritic spines.24
Dopamine
Dopamine is a neurotransmitter which regulates movement and speech. Parkinson’s Disease motor deficits are a result of a loss in dopamine-secreting (dopaminergenic) neurons.
Mice which overexpress A53T alpha synuclein experience impaired dopamine signaling before neurodegeneration,20 and rats expressing A53T alpha synuclein showed bigger losses of dopaminergic neurons than their counterparts expressing WT alpha synuclein.21 It is also thought that A53T alpha synuclein expression makes cells more vulnerable to dopamine toxicity.22
Astrocytes
When A53T alpha synuclein is expressed selectively in mouse astrocytes, it results in rapid paralysis.29 This is thought to be because of decreased glutamate transporter expression disruption resulting in downstream activation of microglia.29
Microglia
Microglia respond to injuries by producing inflammatory mediators and are responsible for synaptic pruning.26 Activated microglia generate reactive oxygen species (ROS), alter morphologies, and release inflammatory cytokines.27
They may become overactivated in Parkinson’s Disease, and help towards the depletion of dopaminergic neurons.27 A53T alpha synuclein activates microglia better than WT or other mutants, resulting in microgliosis and a subsequent inflammatory state.27 A53T alpha synuclein expression also makes cells more vulnerable to oxidative stress.28
Mitochondrial Dysfunction and Endoplasmic Reticulum Stress
A53T alpha synuclein-induced inflammation and oxidative stress affect both the endoplasmic reticulum (ER) and the mitochondria. A53T expression causes mitochondrial dysfunction involving impaired respiratory function and mitochondrial membrane potential.30
In turn, this results in mitochondrial autophagy.31 Overactivation of autophagy may cause mitochondrial loss and resultant neurodegeneration.31 Yet, by clearing aggregated proteins it may also possess some protective effects.31 A53T neurons exhibited impaired mitochondrial motility which could be reversed by an autophagy inducer, rapamycin.30
Increasing A53T alpha synuclein expression resulted in up to 40 % cell death in differentiated PC12 cells. This was due to increased intracellular ROS level and decreased proteasome activity.32
In this instance, mitochondrial function and ER stress involved the release of mitochondrial cytochrome C and elevated caspase-3, -9, and -12 activities, and led to cell death.32
Therapeutic Approaches
Impeding the neurodegeneration linked with A53T mutation could spotlight potential PD therapies. A COX-1 inhibitor prolongs the lifespan of A53T alpha synuclein-expressing mice,29 and Cyclosporin A, Z-Vad, and Caspase-3 and -9 inhibitors decreased cell death from ER and mitochondrial stress.32
Although in the majority of PD cases the A53T mutation is not present, WT alpha synuclein may have slower but similar mechanisms of fibrillization and subsequent neurodegeneration.
A53T mutated alpha synuclein was more successful than WT alpha synuclein in determining a PD model in rats, and lead to a model representative of early-onset PD.21 A better understanding of A53T alpha synuclein fibrillization and inhibition mechanisms could aid those with the A53T mutation in addition to other PD patients.
StressMarq has just launched two A53T mutant alpha synuclein proteins for neurodegenerative disease research: monomers and preformed fibrils.
References and Further Reading
- Fauvet B, et al. alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287:15345–64.
- Dehay B, Bourdenx M, Gorry P, et al. Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol. 2015;14(8):855-866.
- Karpinar DP, et al. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson’s disease models. EMBO J. 2009;28:3256–68.
- Winner B, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–9.
- Cremades N, et al. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. 2012;149:1048–59.
- Danzer KM, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–32.
- Tanaka M, et al. Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. J Biol Chem. 2004;279:4625–31.
- Polymeropoulos, M. H. Mutation in the -Synuclein Gene Identified in Families with Parkinson’s Disease. Science, 1998;276(5321), 2045–2047. doi:10.1126/science.276.5321.2045
- Ki C.S. Stavrou E.F. Davanos N. Lee W.Y. Chung E.J. Kim J.Y. Athanassiadou A. The Ala53Thr mutation in the alpha-synuclein gene in a Korean family with Parkinson disease. Clin Genet. 2007 May;71(5):471-3.
- Conway, K.E., Harper, J.D., & Lansbury, P.T. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat Med. 1998, 4(11):1318-20
- Flagmeier, P. et al. (2016). Mutations associated with familial Parkinson’s disease alter the initiation and amplification steps of α-synuclein aggregation. PNAS. 113(37):10328-10333.
- Lashuel, H.A., Petre, B.M, Wall, J. et al. α-Synuclein, Especially the Parkinson’s Disease-associated Mutants, Forms Pore-like Annular and Tubular Protofibrils. J Mol Biol. 2002 Oct 4;322(5):1089-102
- Coskuner, O., Wise-Scira, O. Structures and Free Energy Landscapes of the A53T Mutant-Type α‑Synuclein Protein and Impact of A53T Mutation on the Structures of the Wild-Type α‑Synuclein Protein with Dynamics. ACS Chem. Neurosci. 2013, 4, 1101−
- Russel, R., Eliezer, D. Residual structure and dynamics in Parkinson’s disease-associated mutants of alpha-synuclein. J Biol Chem. 2001 Dec 7;276(49):45996-6003.
- Guerrero-Ferreira, Taylor NM, Mona D, Ringler P, Lauer ME, Riek R, Britschgi M, Stahlberg H. Cryo-EM structure of alpha-synuclein fibrils. Elife. 2018 Jul 3;7. pii: e36402
- Li, Y. et al. Amyloid fibril structure of α-synuclein determined by cryoelectron microscopy. Cell Research (2018) 0:1–7
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ et al. (2002) Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 34: 521-533.
- Galvagnion C, et al. (2015) Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation. Nat Chem Biol 11(3):229–234.
- Volles, M., Lansbury, P.T. Zeroing in on the Pathogenic Form of α-Synuclein and Its Mechanism of Neurotoxicity in Parkinson’s Disease. Biochem. 2003, 42(26). 10.1021/bi030086j
- Kurz A, Double KL, Lastres-Becker I, Tozzi A, Tantucci M, et al. (2010) A53T-Alpha-Synuclein Overexpression Impairs Dopamine Signaling and Striatal Synaptic Plasticity in Old Mice. PLoS ONE 5(7): e11464.
- Lu, J., Sun, F. et al. (2015). Comparison between alpha synuclein wild-type and A53T mutation in a progressive PD model. Biochem & Biophys Res. Commun. 464(4):988-993.
- Tabrizi, S. J.; Orth, M.; Wilkinson, J. M.; Taanman, J. W.; Warner, T. T.; Cooper, J. M.; Schapira, A. Expression of mutant alpha-synuclein causes increased susceptibility to dopamine toxicity. Mol. Genet. 2000, 9, 2683-2689
- Lepeta K, Lourenco MV, Schweitzer BC, et al. Synaptopathies: synaptic dysfunction in neurological disorders – A review from students to students. J Neurochem. 2016;138(6):785-805.
- Nemani VM, Lu W, Berge V, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. 2010;65(1):66-79.
- Teravskis, P.J. et al. (2018). A53T mutant alpha-synuclein induces tau dependent postsynaptic impairment independent of neurodegenerative changes. Neurosci; 10.1523
- Joe EH, Choi DJ, An J, Eun JH, Jou I, Park S. Astrocytes, Microglia, and Parkinson’s Disease. Exp Neurobiol. 2018;27(2):77-87.
- Hoenen C, Gustin A, Birck C, Kirchmeyer M, Beaume N, Felten P, et al. (2016) Alpha-Synuclein Proteins Promote Pro-Inflammatory Cascades in Microglia: Stronger Effects of the A53T Mutant. PLoS ONE 11(9): e0162717
- Kanda, S.; Bishop, J. F.; Eglitis, M. A.; Yang, Y.; Mouradian, M. M. Enhanced vulnerability to oxidative stress by alpha-synuclein mutations and C-terminal truncation. Neuroscience 2000, 97, 279-284.
- Gu, X., Long, C. et al. (2010). Astrocytic expression of Parkinson’s disease-related A53T α-synuclein causes neurodegeneration in mice. Molecular Brain. 3:12.
- Li L, Nadanaciva S, Berger Z, Shen W, Paumier K, et al. (2013) Human A53T α-Synuclein Causes Reversible Deficits in Mitochondrial Function and Dynamics in Primary Mouse Cortical Neurons. PLoS ONE 8(12): e85815
- Choubey V, Safiulina D, Vaarmann A, et al. Mutant A53T alpha-synuclein induces neuronal death by increasing mitochondrial autophagy. J Biol Chem. 2011;286(12):10814-24.
- Smith, W.W. et al. (2005). Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Gen. 14(24):3801-3811.
Acknowledgments
Produced from materials originally authored by Patricia Thomson from StressMarq Biosciences Inc.
About StressMarq Biosciences
Established in 2007, StressMarq Biosciences Inc. is a supplier of life science products that operates out of Victoria, Canada with a small, but dedicated group of scientists. Headed by our CEO and President Dr. Ariel Louwrier, StressMarq provides the research community with high-quality reagents backed with rigorous quality control data, expert scientific support, and fast international delivery.
“Discovery through partnership, Excellence through quality”
With over 7,000 products, our growth can be attributed to the continual production of cutting edge research products. Our diverse portfolio of primary antibodies, antibody conjugates, proteins, immunoassay kits and small molecules bridges across the life sciences, including products for cancer research, cardiovascular disease, cell signaling and neuroscience. To aid research worldwide, StressMarq has an extensive network of international distributors that allow us to supply reagents to over 50 countries.
In the years to come, StressMarq will continue to aid life science research by providing “Discovery through partnership, and Excellence through quality”.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.