As opposed to a monomer, which has only one unit, or a polymer, which has many units, an oligomeric protein is made up of a few repeating units. Oligo – is derived from the Ancient Greek word meaning “few” and mer comes from the Ancient Greek word for “part.”
Alpha synuclein is a protein involved in Parkinson’s Disease (PD), a common neurodegenerative disorder. While alpha synuclein is usually found in the presynaptic terminals of neurons, it aggregates to form larger fibrils and aggregates called Lewy bodies in certain diseases, known as synucleinopathies.
It forms intermediate species called oligomers during this aggregation process. These oligomers are of interest because rather than Lewy bodies, they are increasingly thought to be the toxic species that result in neuron death in synucleinopathic diseases.1,2
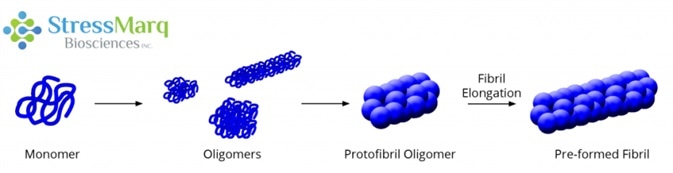
Alpha synuclein monomers can aggregate to form a variety of oligomeric species. If aggregation continues, they form protofibrils and fibrils as found in Lewy bodies. Image Credit: StressMarq Biosciences
How Dopamine Induces Alpha Synuclein Oligomerization
Dopamine is a neurotransmitter which can prevent the aggregation of alpha synuclein into fibrils, stabilizing it in its oligomeric form. Dopamine oxidation is thought to be part of this process.
Dopamine Oxidation Mechanism
The oxidation of the catechol ring to form dopamine-quinone is the first step in dopamine oxidation. This species is then able to react to form dopaminochrome, which then can be converted into 5, 6-indoloquinone and ultimately, neuromelanin.3
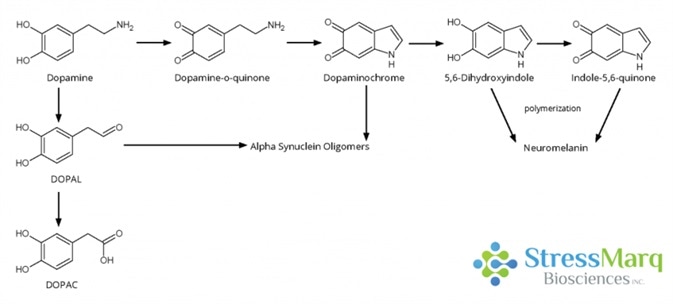
Dopamine oxidation mechanism. Both dopaminochrome and DOPAL are thought to interact with alpha synuclein in a way that promotes oligomer formation. Image Credit: StressMarq Biosciences
Interactions with Alpha Synuclein: Oligomer Stabilization
Through interactions in different regions, dopamine and its derivatives are thought to inhibit alpha synuclein aggregation and kinetically stabilize oligomers.
C-terminus: Y125EMPS129
Computational modeling suggests that the aromatic ring of dopamine interacts with this 5-amino acid sequence of alpha synuclein in a non-covalent, non-specific, hydrophobic way.4 Yet, there is also evidence that the oxidation of the methionine residue in this region, and not binding with dopamine, prevents fibrillization.5
This motif is needed for oligomer stabilization in any case,6-9 and the mutation of Y125EMPS129 to F125AAFA129 stops this by inhibiting interaction with dopamine.7,11
NAC Region: E83
It is thought that dopamine derivatives also have long-range electrostatic interactions with E83 in the NAC region.4 The mutation E83A hinders dopamine’s ability to prevent fibril formation.4
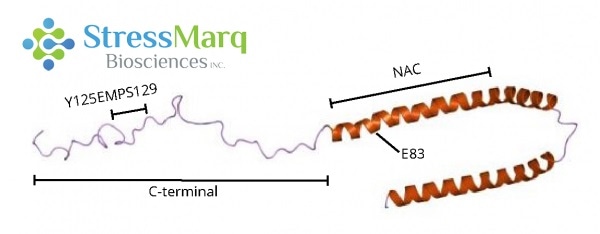
Image Credit: StressMarq Biosciences
Oligomerization Mechanism
Dopamine and its derivatives not only kinetically stabilize oligomers, but in addition, they can induce oligomerization of monomers. Dopamine can prompt dimerization, with residues 43 through 60 in the N-terminal forming the dimer interface.10
Dopamine also induces trimerization, where monomers overlap but do not have associate end-to-end.11 By cross-linking with dopamine-quinone and dopamine-melanin, the oxidation of four methionine residues at the C and N-terminals inhibits end-to-end associations and beta-sheet formation.11
Dopaminochrome can prompt reversible conformational alterations which promote oligomer formation12 and DOPAL, which is a toxic dopamine metabolite, is thought to induce monomer aggregation by cross-linking alpha synuclein lysine residues.13
Alpha Synuclein Oligomer Structure
There are a number of types of alpha synuclein oligomers, both synthesized in labs, and found in living systems. Alpha synuclein oligomers which are derived from post-mortem brains can range from dimers (two units) to 150 units.1,14
Evidence for Oligomer Toxicity
Increasingly, alpha synuclein oligomers are recognized as a toxic species in synucleinopathies. In vitro experiments and animal models have shown their toxicity, as cell death and degeneration happens when there are oligomers are present, even in the absence of large alpha synuclein aggregates.15-18
Yet, there are also non-toxic types of oligomers. The most toxic oligomers are thought to be between 2 and 6 nm in diameter,14 whereas larger oligomers are thought to be non-toxic but able to seed.1
Alpha Synuclein Oligomer Toxicity Mechanisms
Through various mechanisms, alpha synuclein oligomers can be toxic to cells:
Interactions with Lipid Membranes and Pore Formation
Alpha synuclein oligomers have exposed hydrophobic surfaces19 and accessible N-terminal regions which allow them to interact with and destabilize lipid membranes.20 Small, annular oligomers are likely to form pores in vesicle and plasma membranes.21-25
Since hydrophobic oligomers can bind more tightly to organelles and membranes, oligomer hydrophobicity is thought to be linked with toxicity.26 This would account for the reduced toxicity in larger aggregates with fewer accessible hydrophobic patches.
Mitochondrial Dysfunction
By inducing a slow and progressive increase in NADH, alpha synuclein oligomers can cause mitochondrial dysfunction.30 This is linked with increased production of reactive oxygen species, permeability transition pore (PTP) opening, mitochondrial depolarization, inhibition of complex I, and decreased electron flow through the electron transport chain.30
Endoplasmic Reticulum (ER) Stress
Alpha synuclein oligomers can form within the ER lumen and accumulate within the ER, heightening neuronal sensitivity to ER stress.31 This ER stress contributes to neurodegeneration.
Calcium Influx
Via N-type voltage-dependent Ca2+, alpha synuclein oligomers can induce Ca2+ influx.27 It is thought that this process is mediated by the NAC region.28 While alpha synuclein monomers can also influence calcium signaling, only oligomers cause cell death.29
SNARE Complex Inhibition
Large intracellular dopamine-stabilized alpha synuclein oligomers can prevent the formation of the SNARE complex, which decreases the release of neurotransmitters.33
Glutamatergic Receptors
Globular oligomers can change the function of glutamatergic receptors, which results in disrupted neuronal function.31,32
Alpha Synuclein Oligomer Seeding
Different varieties of oligomers exhibit different seeding capacities. Oligomers with higher seeding capacities are linked with lower toxicities.34 Intracellular alpha synuclein oligomers can be released via exosomes, where it is more likely that they are taken up by neighboring cells than free extracellular alpha synuclein oligomers.35
Dopamine-Stabilized Alpha Synuclein Oligomers
Dopamine-induced oligomers have been revealed to auto-aggregate36 and can cross-seed amyloid-beta aggregates.37 Dopamine-induced oligomers have also caused neurodegeneration in mouse and worm in vivo models.38
Yet, it is worth noting that different experimental conditions generate different varieties of oligomers, and the toxicity depends on oligomer synthesis structure and technique. StressMarq has just launched commercially available dopamine-stabilized oligomers which can be used for alpha synuclein research.
As they exhibit potential as a biomarker, oligomers are of interest to researchers as they are present in CSF and plasma, in addition to a treatment target since they are a cause of neurodegeneration.31
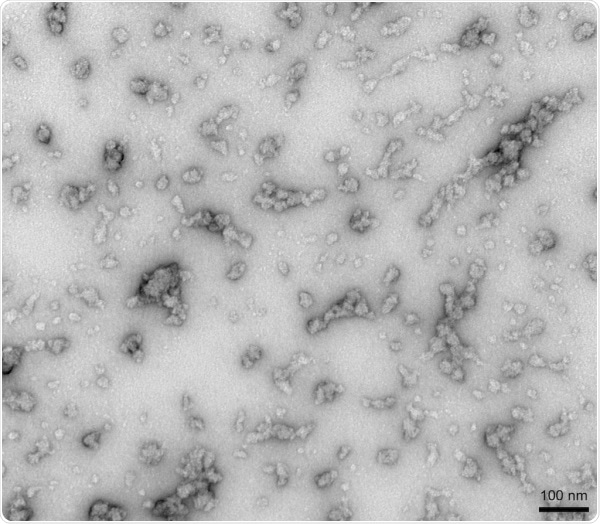
TEM of Human Recombinant Alpha Synuclein Oligomers (Dopamine HCL Stabilized) (SPR-466). Image Credit: StressMarq Biosciences
References and Further Reading
- Danzer, K.M., Haasen, D., Karow, A.R., Moussaud, S., Habeck, M., Giese, A., Kretzschmar, H., Hengerer, B., Kotska, M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007; 27:9220–9232.
- Wright, J.A., Wang, X., Brown, D.R. Unique copper-induced oligomers mediate alpha-synuclein toxicity. FASEB J. 2009; 23(8):2384-93.
- Van Diggelen, F., Tepper, A.W.J.W., Apetri, M.M., Otzen, D.E. α-Synuclein oligomers: a study in diversity. Israel J Chem. 2016; 57(7).
- Herrera, F.E., Chesi, A., Paleologou, K.E., Schmid, A., Muniz, M., Vendruscolo, M., Gustincich, S., Lashuel, H.A., Carloni, P. Inhibition of a-Synuclein Fibrillization by Dopamine Is Mediated by Interactions with Five C-Terminal Residues and with E83 in the NAC Region. PLoS ONE. 2008; 3(10):e3394.
- Leong, S.L., Pham C.L., Galatis, D., Fodero-Tavoletti, M.T., Perez, K., Hill, A.F., et al. Formation of dopamine-mediated alpha-synuclein-soluble oligomers requires methionine oxidation. Free Rad Biol Med. 2009; 46(10):1328–37.
- Mazzulli, J.R., Mishizen, A.J., Giasson, B.I. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci. 2006; 26:10068–10078.
- Mazzulli, J.R., Armakola, M., Dumoulin, M., Parastatidis, I., Ischiropoulos, H. Cellular oligomerization of alpha-synuclein is determined by the interaction of oxidized catechols with a C-terminal sequence. J Biol Chem. 2007; 282:31621–31630.
- Conway, K.A., Rochet, J.C., Bieganski, R.M., Lansbury, P.T. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001; 294:1346–1349.
- Norris, E.H., Giasson, B.I., Hodara, R. Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem. 2005; 280:21212–21219.
- Leong, S.L., Hinds, M.G., Connor, A.R., Smith, D.P., Illes-Toth, E., Pham, C.L., Barnham, K.J., Cappai, R. The N-terminal residues 43 to 60 form the interface for dopamine mediated α-synuclein dimerisation. PLoS One. 2015; 10(2):e0116497.
- Rekas, A., Knott, R.B., Sokolova, A., Barnham, K.J., Perez, K.A., Masters, C.L., Drew, S.C., Cappai, R., Curtain, C.C., Pham, C.L. The structure of dopamine induced alpha-synuclein oligomers. Eur Biophys J. 2010; 39(10):1407-19.
- Norris, E.H., Giasson, B.I., Ischiropoulos, H., Lee, V.M. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J Biol Chem. 2003; 278(29):27230-40.
- Werner-Allen, J.W., Dumond, J.F., Levine, R.L., Bax, A. Toxic Dopamine Metabolite DOPAL Forms an Unexpected Dicatechol Pyrrole Adduct with Lysines of α-Synuclein. Angew Chem Int Ed Eng. 2016; 55(26):7374-8.
- Cremades N, et al. Direct Observation of the Interconversion of Normal and Toxic Forms of alpha-Synuclein. Cell. 2012; 149:1048–1059
- Lee, H.J., Bae, E.J., Lee, S.J. Extracellular α–synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014; 10(2):92-8.
- Xu, J., Kao, S.Y., Lee, F.J., Song, W., Jin, L.W., Yanker, B.A. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 2002; 8(6):600-6.
- Van der Putten, H., Wiederhold, K.H., Probst, A., Barbieri, S., Mistl, C., Danner, S., Kauffman, S., Hofele, K., Spooren, W.P., Ruegg, M.A., Lin, S., Caroni, P., Sommer, B., Tolnay, M., Bilbe, G. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000; 20(16):6021-9.
- Lo Bianco, C., Ridet, J.L., Schneider, B.L., Deglon, N., Aebischer, P. alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc Natl Acad Sci USA. 2002; 99(16):10813-3.
- Van Rooijen, B.D., Claessens, M.M.A.E., Subramaniam, V. Lipid bilayer disruption by oligomeric α-synuclein depends on bilayer charge and accessibility of the hydrophobic core. Biochim Biophys Acta Biomem. 2009; 1788(6):1271-1278.
- Fusco, G., Chen, S.W., Williamson, P.T.F., Cascella, R., Perni, M., Jarvis, J.A., Cecchi, C., Vendruscolo, M., Chiti, F., Cremades, N., Ying, L., Dobson, C.M., De Simone, A. Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers. 2017; 358(3639):1440-1443.
- Volles, M.J., Lee, S.J., Rochet, J.C. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s Disease. Biochem. 2001; 40:7812-7819.
- Ding, T.T., Lee, S.J., Rochet, J.C., Lansbury, P.T. Annular alpha-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochem. 2002; 41:10209-10217.
- Gosavi, N., Lee, H.J., Lee, J.S., Patel, S., Lee, S.J. Golgi Fragmentation Occurs in the Cells with Prefibrillar α-Synuclein Aggregates and Precedes the Formation of Fibrillar Inclusion. J Biol Chem. 2002; 277:48984-48992.
- Lashuel, H.A., Petre, B.M., Wall, J., Simon, M., Nowak, R.J., Walz, T., Lansbury, P.T. Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002; 322(5):1089-102.
- Mosharov, E.V., Staal, R.G., Bove, J., Prou, D., Hananiya, A., Markov, D., Poulsen, N., Larsen, K.E., Moore, C.M., Troyer, M.D., Edwards, R.H., Przedborski, S., Sulzer, D. Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J Neurosci. 2006; 26(36):9304-11.
- Lee, J.E., Sang, J.C., Rodrigues, M., Carr, A.R., Horrocks, M.H., De, S. Bongiovanni, M.N., Flagmeier, P., Dobson, C.M., Wales, D.J., Lee, S.F., Klenerman, D. Mapping Surface Hydrophobicity of α-Synuclein Oligomers at the Nanoscale. Nano Lett. 2018; 18(12):7497-7501.
- Adamczyk, A., Strosznajder, J.B. Alpha-synuclein potentiates Ca2+ influx through voltage-dependent Ca2+ channels. Neuroreport. 2006; 17(18):1883-6.
- Butler, B., Sambo, D., Khoshbouei, H. Alpha-synuclein modulates dopamine neurotransmission. J Chem Neuroanat. 2017; 83-84:41-49.
- Angelova, P.R., Ludtmann, M.H., Horrocks, M.H., Negoda, A., Cremades, N., Klenerman, D., Dobson, C.M., Wood, N.W., Pavlov, E.V., Gandhi, S., Abramov, A.Y. Ca2+ is a key factor in α-synuclein-induced neurotoxicity. J Cell Sci. 2016; 129(9):1792-801.
- Ludtmann, M.H.R., Angelova, P.R., Horrocks, M.H., Choi, M.L., Rodrigues, M., Baev, A.Y., Berezhnov, A.V., Yao, Z., Little, D., Banushi, B., Al-Menhali, A.S., Ranasinghe, R.T., Whiten, D.R., Ratsuda, Y. Dolt, K.S., Devine, M.J., Gissen, P. Kunath, T., Jaganjac, M., Pavlov, E.V., Klenerman, D., Abramov, A.Y., Gandhi, S. α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun. 2018; 9:2293.
- Kalia, L.V., Kalia, S.K., McLean, P.J., Lozano, A.M., Lang, A.E. α-Synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol. 2013; 73(2):155-169.
- Diogenes, M.J., Dias, R.B., Rombo, D.M., Vincente Miranda, H., Maiolino, F., Guerreiro, P., Nasstrom, T., Franquelim, H.G., Oliveira, L.M., Castanho, M.A., Lannfelt, L., Bergstrom, J., Ingelsson, M., Quintas, A., Sebastiao, A.M., Lopes, L.V., Outeiro, T.F. Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation. J Neurosci. 2012; 32(34):11750-62.
- Choi, B.K., Choi, M.G., Kim, J.Y., et al. Large alpha-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci USA. 2013; 110:4087–4092.
- Mor, D.E., Ischiropoulos, H. The Convergence of Dopamine and α-Synuclein: Implications for Parkinson’s Disease. J Exp Neurosci. 2018; 20(11):1560-1568.
- Danzer, K.M., Kranich, L.R., Ruf, W.P., Cagsal-Getkin, O., Winslow, A.R., Zhu, L., Vanderburg, C.R., McLean, P.J. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Molecular Neurodegeneration. 2012; 7:42.
- Pieri, L, Madiona, K., Melki, R. Structural and functional properties of prefibrillar α-synuclein oligomers. Sci Rep. 2016; 6:24526.
- Planchard, M.S., Exley, S.E., Morgan, S.E., Rangachari, V. Dopamine-induced α-synuclein oligomers show self- and cross-propagation properties. Protein Sci. 2014; 23(10):1369-79.
- Mor, D.E., Tsika, E., Mazzulli, J.R., Gould, N.S., Kim, H., Daniels, M.J., Doshi, S., Gupta, P., Grossman, J.L., Tan, V.S., Kalb, R.G., Caldwell, K.A., Caldwell, G.A., Wolfe, J.H., Ischiropoulos, H. Dopamine induces soluble α-synuclein oligomers and nigrostriatal degeneration. Nat Neurosci. 2017; 20(11):1560-1568.
Acknowledgments
Produced from materials originally authored by Patricia Thomson from StressMarq Biosciences Inc.
About StressMarq Biosciences
Established in 2007, StressMarq Biosciences Inc. is a supplier of life science products that operates out of Victoria, Canada with a small, but dedicated group of scientists. Headed by our CEO and President Dr. Ariel Louwrier, StressMarq provides the research community with high-quality reagents backed with rigorous quality control data, expert scientific support, and fast international delivery.
“Discovery through partnership, Excellence through quality”
With over 7,000 products, our growth can be attributed to the continual production of cutting edge research products. Our diverse portfolio of primary antibodies, antibody conjugates, proteins, immunoassay kits and small molecules bridges across the life sciences, including products for cancer research, cardiovascular disease, cell signaling and neuroscience. To aid research worldwide, StressMarq has an extensive network of international distributors that allow us to supply reagents to over 50 countries.
In the years to come, StressMarq will continue to aid life science research by providing “Discovery through partnership, and Excellence through quality”.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.