Alpha synuclein is a key drug target in Parkinson's Disease with the ability to aggregate into oligomers and fibrils. Research into α-synuclein has rapidly progressed in recent years with a lot of the work focussing on the use of pre-formed recombinant fibrils (PFFs).
It has since become apparent, however, that there are a number of different fibril preparations and each have quite different properties. This article lists and examines some fibril and oligomer preparations, along with other useful tools for alpha synuclein research.
Monomers (Types 1 and 2)
There are two possible ways to make α-Synuclein monomers, yielding Type 1 and 2 materials. Any fibrillar or oligomeric material larger than 30kD is removed by filtering both of these. Unlike with Type 2 monomers (SPR-316), fibrils with a high Thioflavin T response are generated with Type 1 monomers (SPR-321) (Figure 1).
Both of these, however, form insoluble fibrils upon aggregation. Fibrillar or oligomeric α-synuclein containing experiments can make use of monomeric α-synuclein as a control. Type 2 monomers (SPR-316) are being examined for use in RT-QuIC assays.
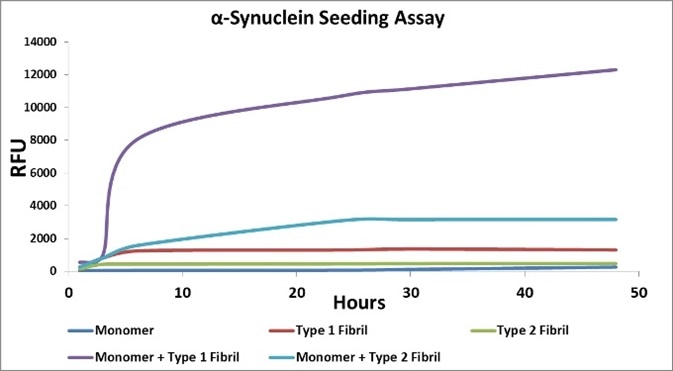
Figure 1. Image Credit: StressMarq Biosciences
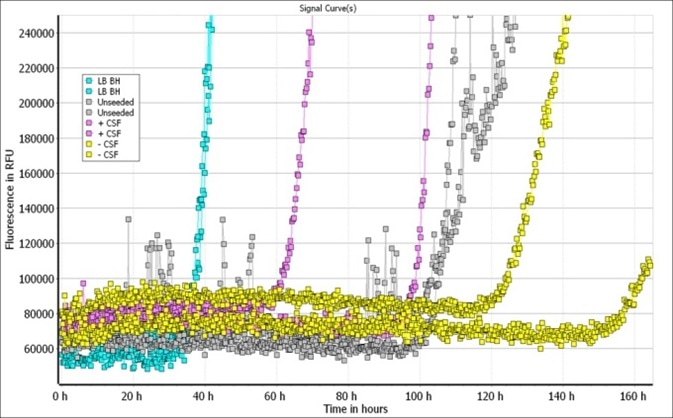
Figure 2. Image Credit: StressMarq Biosciences
Fibrils (Types 1, 2 and 3) and Filaments
Type 1 and 2 monomers are aggregated to generate Type 1 (SPR-322) and 2 (SPR-317) fibrils, respectively, under specific conditions. However, the endotoxin content of Type 1 fibrils (approximately 10-20 EU/ml) has led to the development of Type 3 fibrils (SPR-448) using an artificial, removable scaffold that allows the building of high Thioflavin T-absorbing fibrils. Whilst the essential properties are the same as Type 1 fibrils, endotoxin content is lower, (2 EU/ml or less).
Filaments (SPR-450) are likely a mixture of soluble proto-fibrils. EM images of Type 1-3 fibrils and filaments are shown in Figure 3. It has been demonstrated that Type 1 human and mouse fibrils induce phospho-serine129 pathology in rat neurocortical primary cells (Figure 4) and in vivo (Figure 5). Furthermore, fluorescently-conjugated PFFs were taken up, transported into the soma, and induced α-synuclein aggregation in mouse neurocortical primary cells (Figures 6 and 7).
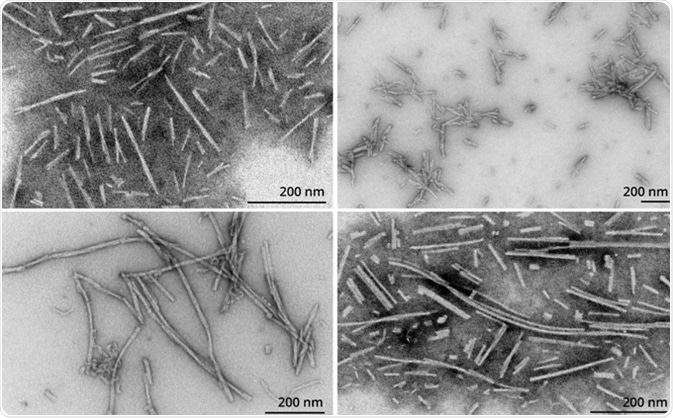
Figure 3. TEM of Type 1 PFFs (SPR-322) (TL); Type 2 PFFs (SPR-317) (TR); Type 3 PFFs (SPR-448) (BL); Filaments (SPR-450) (BR). Image Credit: StressMarq Biosciences
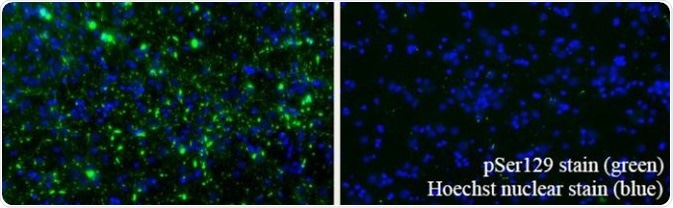
Figure 4. Left: Type 1 PFFs (SPR-322). Right: Type 2 PFFs (SPR-317). Image Credit: StressMarq Biosciences
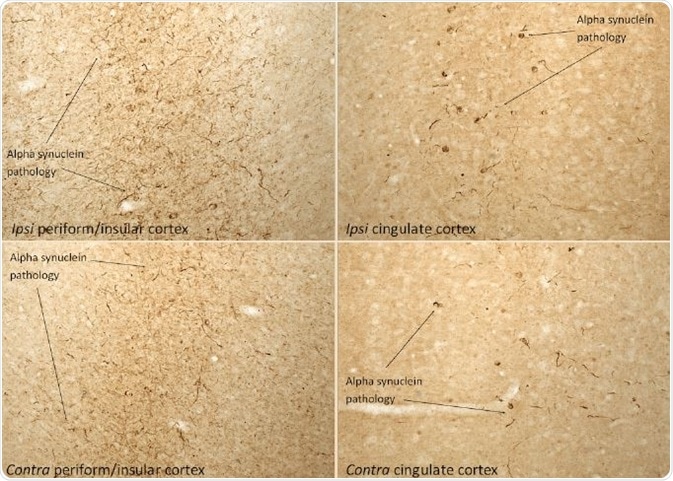
Figure 5. IHC analysis of rat brain injected with Type 1 mouse alpha synuclein PFFs (SPR-324) shows α-synucleinpathology. Image Credit: StressMarq Biosciences
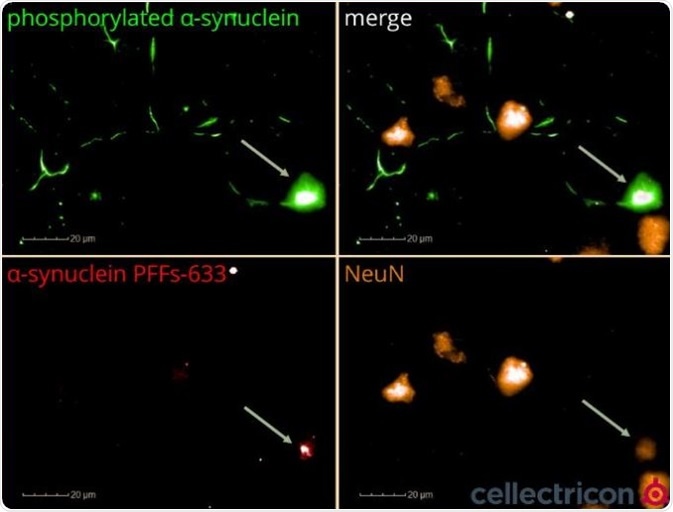
Figure 6. ICC of primary mouse cortical neurons seeded with ATTO633-labelled α-synucleinPFFs (SPR-322). Image Credit: StressMarq Biosciences
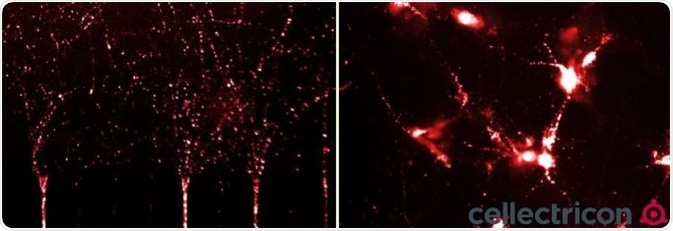
Figure 7. ICC of primary mouse cortical neurons cultured in a microfluidic co-culture system seeded with ATTO633-labelled α-synucleinPFFs (SPR-322). Image Credit: StressMarq Biosciences
α-Synuclein Mutants
There have been studies looking at several mutants of human α-synuclein. The A53T mutant is the best studied of these. This makes the resultant protein more prone to aggregation and fibrillization (Figure 8). The PFFs can be detected with both total ELISA-based α- synuclein assays and FRET-based aggregation assays along with the aggregation process (Figure 9). Phospho-serine129 pathology in rat neurocortical primary cells is also induced by A53T PFFs (Figure 10).
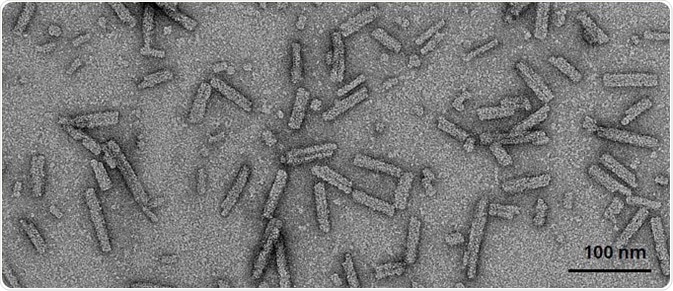
Figure 8. TEM of A53T human α-synuclein PFFs. (SPR-326). Image Credit: StressMarq Biosciences
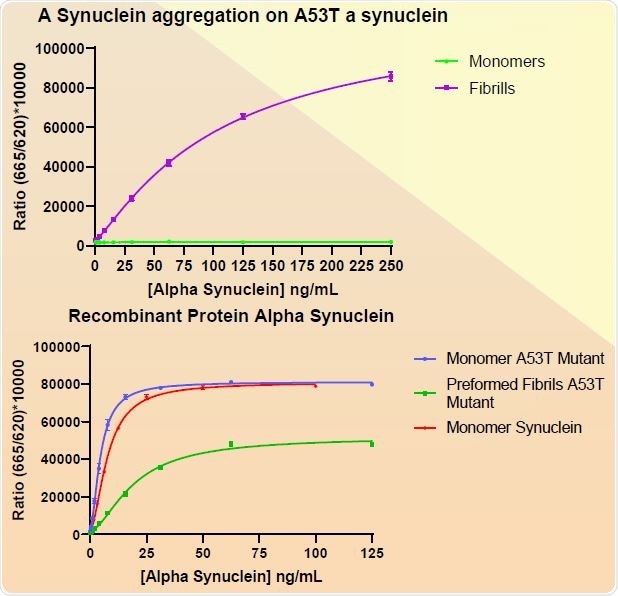
Figure 9. CisbioAggregation (top) and Total α-synucleinassay kits of A53T PFFs (SPR-326). Image Credit: StressMarq Biosciences
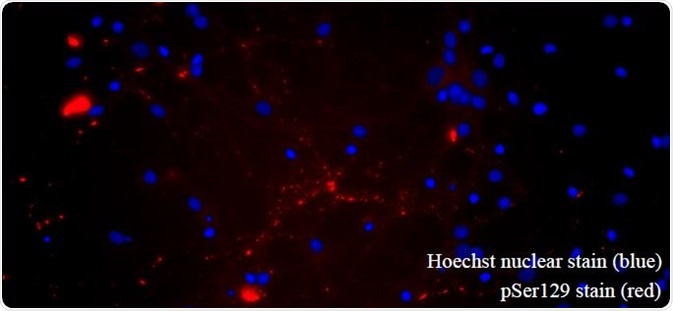
Figure 10. A53T PFFs seed α-synucleinpathology in primary rat hippocampal neurons. (SPR-326). Image Credit: StressMarq Biosciences
α-Synuclein Oligomers
It is now clear that α-synuclein goes through a number of steps to become insoluble PFFs. A number of large and small oligomers are included in these intermediates. There is increasing interest in these as they have seemingly different properties to PFFs and may represent the toxic element associated with these proteins.
In terms of the formation of stabilized and toxic oligomers, dopamine and its oxidized byproducts seem to be instrumental (Figure 11). There are clear morphological differences to soluble filaments or insoluble PFFs, as these are generally circular with a diameter of about 25 nm.
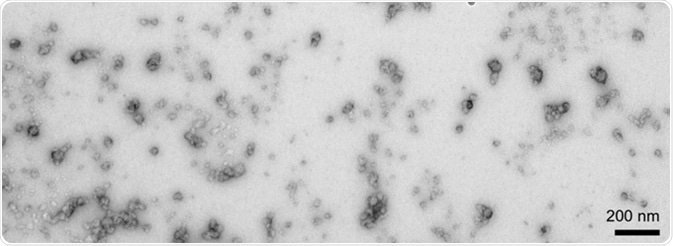
Figure 11. TEM of α-synucleindopamine-stabilized oligomers (SPR-466). Image Credit: StressMarq Biosciences
α-Synuclein Antibodies
In Lewy bodies ninety percent of α-synuclein is phosphorylated at Ser129, meaning that antibodies against it are useful tools for detecting α-synuclein inclusions.
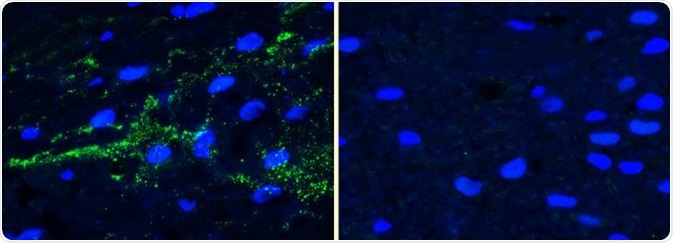
Figure 12. IHC analysis using alpha synucleinpSer129 antibody (SPC-742) of mouse brain injected with alpha synucleinAAV vector (L). Control (R). Image Credit: StressMarq Biosciences
β- and γ-Synuclein, Monomers and Fibrils
Whilst neither have been well studied, α-Synuclein has a high degree of homology with both β- and γ-synuclein. Although γ-synuclein can aggregate and seed reactions, β-synuclein lacks the NAC domain and is not able to readily fibrillize or seed reactions unless chemically forced to do so. This potentially makes it a valuable control for α-synuclein. Both monomeric and fibrillized (Figure 13) versions of these proteins have been generated.
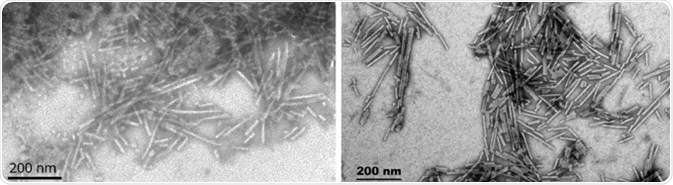
Figure 13. TEM of β-synucleinPFFs (SPR-457) (L) and γ-synucleinPFFs (SPR-459) (R). Image Credit: StressMarq Biosciences
Acknowledgments
All A53T α-synuclein aggregation and detection data using Cisbio Kits was kindly provided by Cisbio, France by Delphine Jaga and Karine Lafargue using StressMarq-provided A53T protein monomers and PFFs. All RT- QuIC data was kindly provided by Alison Green and Graham Fairfoul, University of Edinburgh, UK using StressMarq provided α-synuclein monomers (Type 2).
Primary cell work using Type 1, Type 2, and A53T PFFs carried out by Rehana Leak, Duquesne University, USA. In vivo data was kindly carried out by Atuka Inc., Canada using StressMarq-provided Type 1 PFFs. Testing of StressMarq-provided pSer129 antibody done by Trine Rasmussen and Simon Molgaard Jensen of Aarhus University. Experiments with ATTO633-labelled α-synuclein done by Cellectricon.
Produced from materials originally authored by Alexandra Netter-Glangeaud, Patricia Thomson, Ariel Louwrier, PhD. from StressMarq Biosciences Inc.
About StressMarq Biosciences
Established in 2007, StressMarq Biosciences Inc. is a supplier of life science products that operates out of Victoria, Canada with a small, but dedicated group of scientists. Headed by our CEO and President Dr. Ariel Louwrier, StressMarq provides the research community with high-quality reagents backed with rigorous quality control data, expert scientific support, and fast international delivery.
“Discovery through partnership, Excellence through quality”
With over 7,000 products, our growth can be attributed to the continual production of cutting edge research products. Our diverse portfolio of primary antibodies, antibody conjugates, proteins, immunoassay kits and small molecules bridges across the life sciences, including products for cancer research, cardiovascular disease, cell signaling and neuroscience. To aid research worldwide, StressMarq has an extensive network of international distributors that allow us to supply reagents to over 50 countries.
In the years to come, StressMarq will continue to aid life science research by providing “Discovery through partnership, and Excellence through quality”.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.