Amyloid-beta-focused therapeutics have repeatedly failed in clinical trials meaning that Tau is now particularly of interest in Alzheimer’s Disease.
The study of tau aggregation and pathology in vitro and in vivo is now facilitated through the introduction of new research tools, such as pre-formed fibrils (PFFs). Fibrils, filaments, and other useful tools for tau research are listed and examined in this article.
Types of Tau Fibrils and Filaments
There are six isoforms of tau expressed in the human brain, the longest of which consists of four repeat domains (R) and two amino acid inserts (N), and is 441 amino acids long (Tau441, 2N4R). The truncated fragment, K18, only consists of the four repeat domains (4R). The truncated fragment, dGAE, consists of amino acids 297-391 and fibrillizes in the absence of templates or additives.
Aggregation is enhanced by the mutations P301S, P301L, C322A and ΔK280. Tau can aggregate into large fibrils and filaments, which, in turn, seed further aggregation of monomers in vitro and in vivo. As shown in Figure 1, 2N4R P301S PFFs (SPR-329) and K18 P301L PFFs (SPR-330) were fibrillized using heparin.
Figure 2 shows dGAE PFFs (SPR-461 and SPR-462) and K18 ΔK280 PFFs (SPR-477), which were fibrillized without scaffolds. K18 ΔK280 PFFs (SPR-477) can form paired helical filaments (PHFs) independently. 2N4R P301S soluble paired helical filaments (SPR-463) shown in Figure 3 were made using a linear anionic scaffold which was then removed.
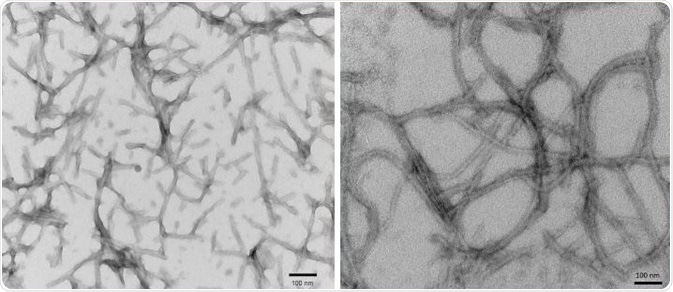
Figure 1. TEM of 2N4R P301S PFFs (SPR-329) (L) and K18 P301L PFFs (SPR-330) (R). Image Credit: StressMarq Biosciences
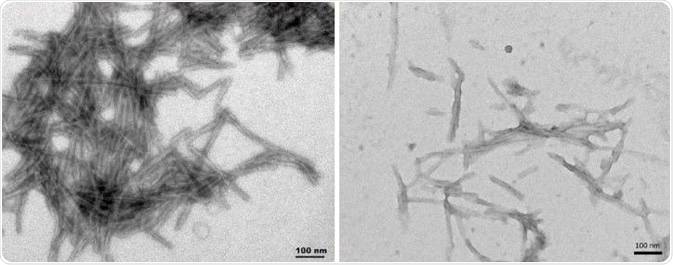
Figure 2. TEM of dGAEWT PFFs (SPR-461) (L), K18 ΔK280 PFFs (SPR-477) (R). Image Credit: StressMarq Biosciences
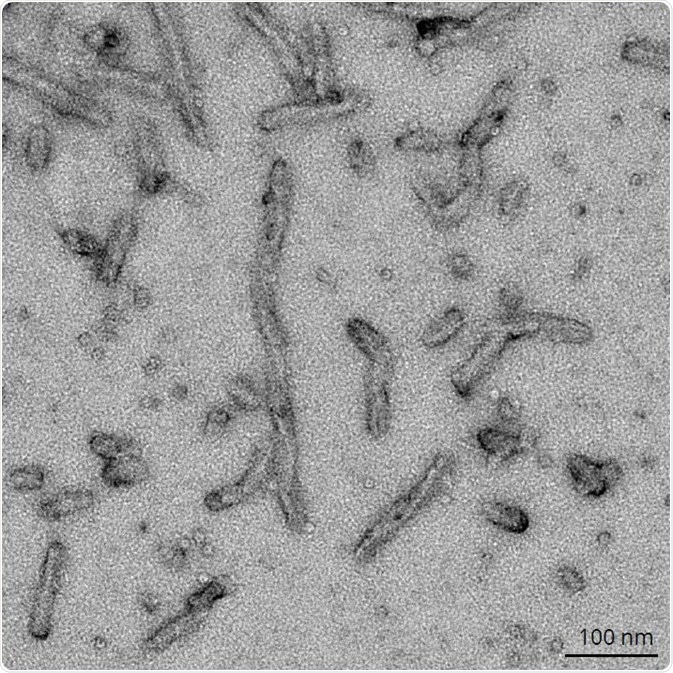
Figure 3. TEM of 2N4R P301S Paired Helical Filaments (SPR-463). Image Credit: StressMarq Biosciences
Tau Aggregation
Tau aggregation into paired helical filaments and neurofibrillary tangles is indicative of tauopathies such as Alzheimer’s Disease. As seen in a thioflavin T fluorescence assay, Tau PFFs can seed the aggregation of tau monomers. A fluorescent dye that binds to beta-sheet-rich structures, such as those in tau fibrils is Thioflavin T.
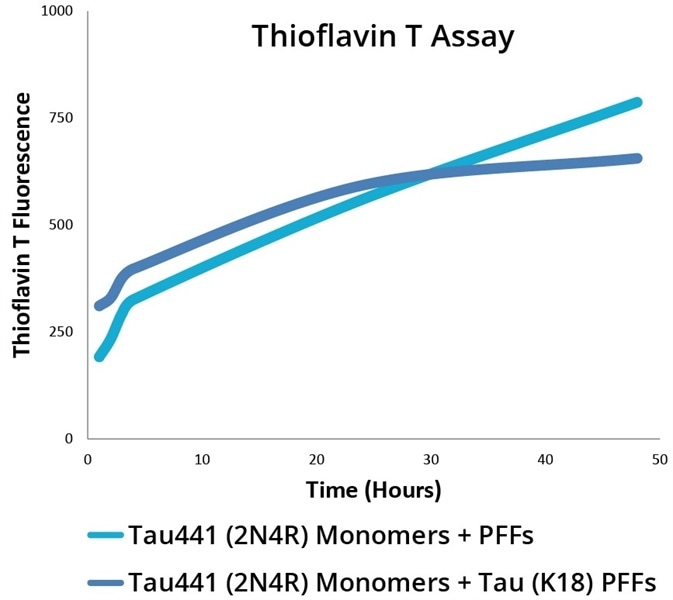
Figure 4. Thioflavin T Fluorescence Assay for 2N4R P301S PFFs (SPR-329) and K18 P301L PFFs (SPR-330) combined with 2N4R P301S monomers (SPR-327). Image Credit: StressMarq Biosciences
Aggregation of tau monomers in solution can be induced by Tau PFFs. But not only this, they can also induce aggregation of tau in vivo. P301L mouse brains had K18 P301L PFFs (SPR-330) injected into them, which seeded tau aggregation in the hippocampus within nine weeks. As shown in Figure 5, this was detected using pSer202/pThr205 tau antibody AT8.
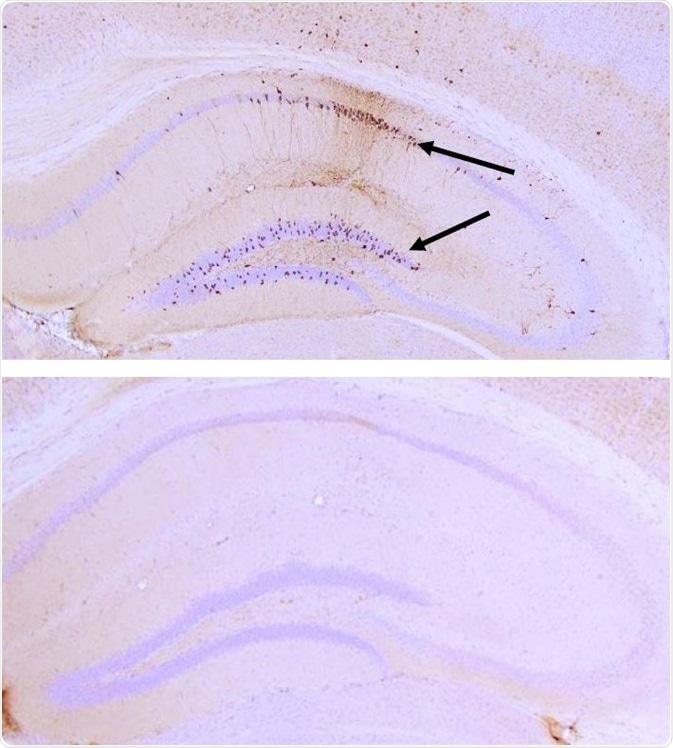
Figure 5. IHC analysis with AT8 of P301L mouse hippocampus after injection with P301L K18 PFFs (SPR-330) (top) and brainstem homogenate (BSH) of non-Tgmice (bottom). AT8 (pSer202/pThr205) tau antibody shows tangle-like inclusions in PFF-injected mice (top) but not in negative control (bottom). Image Credit: StressMarq Biosciences
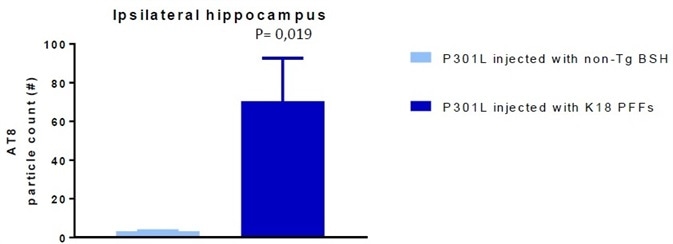
Figure 6. AT8 particle count in P301L mice injected with non-Tgbrainstem homogenate (BSH) (negative control) and K18 P301L PFFs SPR-330). Image Credit: StressMarq Biosciences
Tau Antibodies
Hyperphosphorylated tau is found in paired helical filaments. There is a correlation between tau aggregation and phosphorylation at certain sites, such as serine 202 and threonine 205, which is therefore indicative of pathology.
AH36 (SMC-601) is a rabbit monoclonal antibody that can detect Ser202/Thr205 phosphorylation. As demonstrated in Figure 7, it has been tested in iPSC-derived cortical excitatory neurons with and without the MAPT P301L mutation.
| |
Non-Demented
Control |
P301L MAPT |
| StressMarq Clone AH36 (SMC-601) |
|
| Thermo Fisher Clone AT8 |
|
Figure 7. ICC/IF analysis of iPSC-derived cortical neurons from non-demented control sample (L) and individual with a P301L MAPT mutation (R) using StressMarq Clone AH36 pSer202/pThr205 tau antibody (SMC-601) and ThermoFisher Clone AT8 pSer202/pThr205 tau antibody. Image Credit: StressMarq Biosciences
Acknowledgments
In vivo experiments and imaging (Figures 5 and 6) were completed at reMYND N.V. with StressMarq-provided PFFs.
Produced from materials originally authored by Alexandra Netter-Glangeaud, Patricia Thomson, Ariel Louwrier, PhD. from StressMarq Biosciences Inc.
About StressMarq Biosciences
Established in 2007, StressMarq Biosciences Inc. is a supplier of life science products that operates out of Victoria, Canada with a small, but dedicated group of scientists. Headed by our CEO and President Dr. Ariel Louwrier, StressMarq provides the research community with high-quality reagents backed with rigorous quality control data, expert scientific support, and fast international delivery.
“Discovery through partnership, Excellence through quality”
With over 7,000 products, our growth can be attributed to the continual production of cutting edge research products. Our diverse portfolio of primary antibodies, antibody conjugates, proteins, immunoassay kits and small molecules bridges across the life sciences, including products for cancer research, cardiovascular disease, cell signaling and neuroscience. To aid research worldwide, StressMarq has an extensive network of international distributors that allow us to supply reagents to over 50 countries.
In the years to come, StressMarq will continue to aid life science research by providing “Discovery through partnership, and Excellence through quality”.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.