The NIH-funded ENCODE project is an initiative intended to produce and offer publicly available data associated with the human genome. ENCODE stands for The Encyclopedia of DNA Elements and it is an ongoing project for characterizing all of the functional elements in the mouse and human genomes.
The ENCODE project and its benefits
At present, it is in its fourth round of funding, and the NIH has awarded grants to a group of institutions from all over the United States to perform research as part of this project.
Apart from making the results from ENCODE consortium experiments available to all, the reagents, methods and data processing pipelines employed in ENCODE experiments will also be validated and published.
This helps ensure consistency between ENCODE experiments performed at various sites and creates open resources for all researchers to also enhance the validation of their experiments.
Antibodies are among the reagents validated by ENCODE. Characterization of antibodies is crucial due to the variation in results acquired between antibodies from various manufacturers.
Certain reasons behind this variability are the natural variability that exists between polyclonal antibody preparations, the varying quality control processes between different manufacturers, and modifications to the hybridoma used to synthesize a monoclonal antibody.1
The ENCODE project enforces a rigorous antibody validation process, for which the lot number and manufacturer of each antibody are recorded, as well as the characterization data.
The validation of an antibody requires multiple confirmatory results; immunoblots, immunoprecipitation, knockdowns or knockouts and mass spectrometry are certain assay types that can be employed for this process.
In ENCODE, characterization data includes primary data as well as the method employed to derive the data along with the name of the lab that performed the experiment.
The requirements for the validation of this data include the need for the antibody to detect a protein at the predicted size, with considerations for the protein to be in its fixed, native, or denatured state while the assay is being performed.
Unique characterization requirements for antibodies that detect RNA transcription factors, binding proteins, chromatin-associated proteins, and epitope-tagged proteins must consider the nuances of detecting these specific types of proteins.2
Through ENCODE, antibodies from more than 100 different manufacturers have already been characterized, such as antibodies synthesized by commercial companies and individual labs.
Of all commercial antibody manufacturers, Bethyl holds the highest rate of antibodies that passed validation, where 86% of the 371 Bethyl antibodies tested achieved this standard.
This is more than the 62% average among antibodies synthesized by all other manufacturers, which indicates the rigorous validation and quality control that Bethyl enforces before its antibodies are distributed.
Conclusion
So far, almost 600 antibodies have been characterized in compliance with ENCODE standards and additionally more than 1200 are awaiting validation.
With continuous expansion, the ENCODE database is a crucial resource for the scientific community as it enables researchers to examine the reliability of their antibody source and make an informed decision on where to order future reagents from.
With the further continuation of the ENCODE project, the database will only be more informative and extensive.
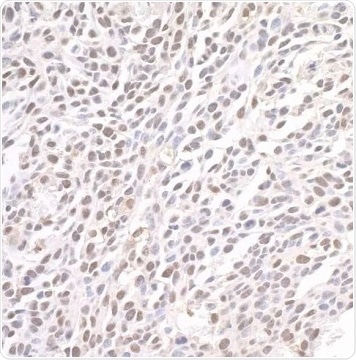
Figure 2. Detection of mouse BRD4 by immunohistochemistry. Sample: FFPE section of mouse CT26 colon carcinoma. Antibody: Affinity purified rabbit anti-BRD4 (Cat. No. A301-985A100 Lot7) used at a dilution of 1:1,000 (1 µg/mL). Detection: DAB. View Bethyl's entire catalog of BRD4-related antibodies. Image Credit: Bethyl Laboratories Inc.
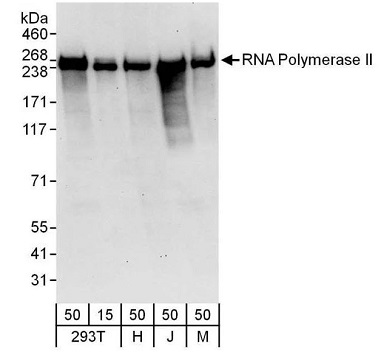
Figure 3. Detection of human and mouse RNA Polymerase II by western blot. Samples: Whole cell lysate from HEK293T (15 and 50 µg for WB), HeLa (H; 50 µg), Jurkat (J; 50 µg) and mouse NIH 3T3 (M; 50 µg) cells. Antibodies: Affinity purified rabbit anti-RNA Polymerase II antibody A300-653A (lot A300-653A-3) used for WB at 0.1 µg/mL. Detection: Chemiluminescence with an exposure time of 30 seconds. View Bethyl's entire catalog of POLR2A-related antibodies. Image Credit: Bethyl Laboratories Inc.
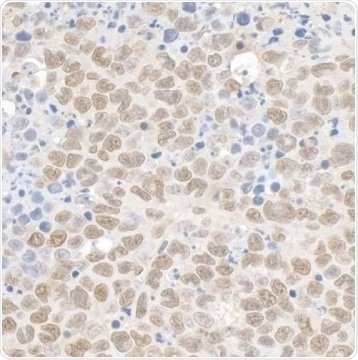
Figure 4. Detection of mouse Kap-1 by immunohistochemistry. Sample: FFPE section of mouse plasmacytoma. Antibody: Affinity purified rabbit anti-Kap-1 Cat. No. A300-275A Lot4 used at a dilution of 1:1,000 (1 µg/mL). Detection: DAB. View Bethyl's entire catalog of TRIM28-related antibodies. Image Credit: Bethyl Laboratories Inc.
References
- Voskuil J (2014) Commercial antibodies and their validation. F1000Research 3:232. doi: 10.12688/f1000research.4966.2.
- Marx V (2019) What to do about those immunoprecipitation blues. Nat Methods 16:289–292. doi: 10.1038/s41592-019-0365-3.
About Bethyl Laboratories, Inc.

Bethyl Laboratories, Inc. has been dedicated to improving lives by supporting scientific discovery through its qualified antibody products and custom polyclonal services since its founding in 1972. Bethyl has a global reputation for quality, consistency and first-class customer care. Every antibody that Bethyl sells is manufactured to exacting standards in Montgomery, Texas, and is validated in-house by a team of scientists. From the veterinary facilities to the development, production, and validation labs, the entire Bethyl team focuses on delivering quality products and delighting customers.
Bethyl Laboratories has been acquired by Fortis Life Sciences. To learn more visit: https://promotions.bethyl.com/news/fortis-life-sciences-acquires-bethyl-laboratories/.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.