Fluorescent labels were conjugated initially to antibodies against targets of interest in the 1940s. Since then, several breakthrough advancements have allowed the development of immunohistochemistry.
The advancements include enzyme digestion and heat-driven methods to epitope retrieval, antibody purification and labeling, enhancements in imaging technologies and signal amplification.
Tyramide signal amplification and its benefits
Specifically, both tyramide signal amplification (TSA) and enhancements in imaging have allowed the advancement of immunohistochemistry over conventional or one-color methods to fluorescent multiplex immunohistochemistry (mIHC).
The advancement of TSA allows signal amplification and imaging of low abundance targets by improving the antigen-associated fluorescence signal.
In combination with the availability of a huge number of fluorophore filters and reporters, this allows a more precise visualization of a huge number of targets of interest inside a single tissue sample. At the same time, enhancements in software and imaging platforms have allowed high quality and data-dense images to be captured.
Regardless of these developments, more extensive use of fluorescent mIHC with TSA is restricted by the time needed to carry out the staining, specifically when several targets of interest are investigated. Process automation might reduce the hands-on time needed to carry out the method and increase throughput.
But such instrumentation is usually cost-intensive and does not avoid the need for considerable time investment up-front in the form of validation and optimization.
Likewise, multispectral imaging of entire slides at high resolution also takes a lot of time. The software and equipment needed to capture high resolution images are also costly, which further restricts the number of users.
Although a majority of the previous utility of IHC was limited to the basic science laboratory, the advancements mentioned in this article have made fluorescent mIHC using TSA into a robust tool for translational research.
Going forward, more technical advancements and more extensive availability and affordability of imaging technology will probably enable mIHC transition to a combined, workhorse clinical tool.
Clinical use will necessitate highly reproducible, measurable outcomes, as well as adherence to regulatory needs. Clinical mIHC applications will probably have to be developed with guidance from organizations like the American Society for Clinical Pathology.
But these applications exhibit huge potential for achieving considerable progress in clinical patient care, such as characterization of the complex tumor microenvironment and a further selection of targeted therapies.
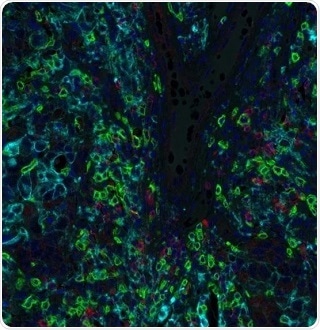
Figure 2. Detection of human CD8 (green) TIGIT (red) and PD-L1 (cyan) in breast carcinoma by IHC-IF. Antibodies: Rabbit anti-CD8 recombinant monoclonal [BLR044F] (A700-044), rabbit anti-TIGIT recombinant monoclonal [BLR047F] (A700-047) and rabbit anti-PD-L1 recombinant monoclonal [BLR020E] (A700-020). Secondary: HRP-conjugated goat anti-rabbit IgG (A120-501P). Substrate: Opal™ 520, Opal™ 690, and Opal™ 480. Counterstain: DAPI (blue). Image Credit: Bethyl Laboratories Inc.
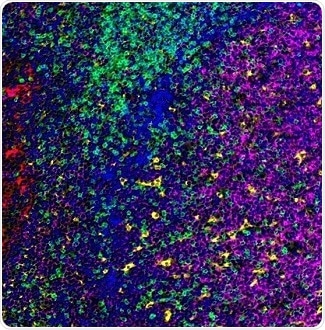
Figure 3. Detection of human CD3 (green), CD20 (magenta), CD68 (yellow), and cytokeratin (red) in FFPE tonsil by IHC-IF. Antibodies: Rabbit anti-CD3E recombinant monoclonal [BL-298-5D12] (A700-016), mouse anti-CD20 monoclonal [L26] (A500-017A), mouse anti-CD68 monoclonal [KP-1] (A500-018A), and mouse anti-Cytokeratin [AE1/AE3] (A500-019A). Secondary: HRP-conjugated goat anti-rabbit IgG (A120-501P) and HRP-conjugated goat anti-mouse IgG (A90-116P). Substrate: Opal™. Counterstain: DAPI (blue). Image Credit: Bethyl Laboratories Inc.
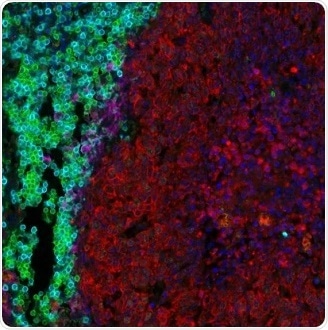
Figure 4. Detection of human CD3 (aqua), CD8 (green), cytokeratin (red), and PD-L1 (magenta) in FFPE lung carcinoma by IHC-IF. Rabbit anti-CD3E recombinant monoclonal [BL-298-5D12] (A700-016), rabbit anti-CD8 alpha [BLR044F] (A700-044), mouse anti-Cytokeratin [AE1/AE3] (A500-019A), and rabbit anti-PD-L1 [BLR020E] (A700-020). Secondary: HRP-conjugated goat anti-rabbit IgG (A120-501P) and HRP-conjugated goat anti-mouse IgG (A90-116P). Substrate: Opal™. Counterstain: DAPI (blue). Image Credit: Bethyl Laboratories Inc.
About Bethyl Laboratories, Inc.

Bethyl Laboratories, Inc. has been dedicated to improving lives by supporting scientific discovery through its qualified antibody products and custom polyclonal services since its founding in 1972. Bethyl has a global reputation for quality, consistency and first-class customer care. Every antibody that Bethyl sells is manufactured to exacting standards in Montgomery, Texas, and is validated in-house by a team of scientists. From the veterinary facilities to the development, production, and validation labs, the entire Bethyl team focuses on delivering quality products and delighting customers.
Bethyl Laboratories has been acquired by Fortis Life Sciences. To learn more visit: https://promotions.bethyl.com/news/fortis-life-sciences-acquires-bethyl-laboratories/.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.