Low oxygen levels in the cellular environment are known to trigger the hypoxic response pathway. Hypoxia-inducible transcription factor, or HIF for short, is fundamental to the hypoxic response.
HIF occurs as a heterodimeric transcription factor made up of an alpha and beta subunit. Mammals have three HIF-alpha subunits - called HIF1-alpha, HIF2-alpha and HIF3-alpha - and also one beta subunit, the aryl hydrocarbon receptor nuclear translocator, abbreviated as ARNT.
When HIF1-alpha and HIF2-alpha are overly expressed, this condition may lead to poor survival rates for different types of cancers.
Clinical and experimental proof strongly indicates that tumor development and treatment response are influenced by HIF1-alpha and HIF2-alpha. Consequently, there has been a great deal of interest in designing selective HIF inhibitors, but because of the complexity of the HIF pathway, this process has proved to be very difficult.
Hence, upcoming studies intended for therapeutic targeting of the HIFs will demand a deeper insight into the HIF1-alpha and HIF2-alpha mechanisms.
Bethyl Laboratories has broadened its capabilities and currently provides rabbit recombinant monoclonal antibodies to both HIF1-alpha and HIF2-alpha. Such antibodies are delivered as a trial size only (labeled in the catalog ending in “-T”) and have been tested for numerous applications.
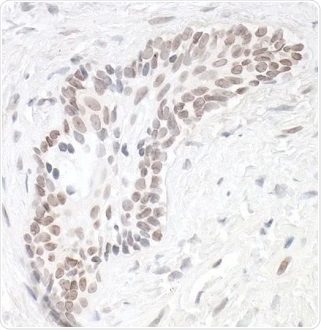
Figure 1. Detection of human HIF1-alpha in FFPE renal cell carcinoma by IHC. Antibody: Rabbit anti-HIF1-alpha recombinant monoclonal [BL-124-3F7] (A700-001). Secondary: HRP-conjugated goat anti-rabbit IgG (A120-501P). Substrate: DAB. Image Credit: Bethyl Laboratories Inc.
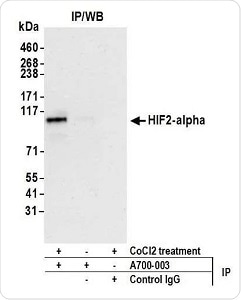
Figure 2. Detection of human HIF2-alpha by WB of immunoprecipitates from HEPG2 lysate treated with 200 µM CoCl2 (+) or mock-treated (−). Antibody: Rabbit anti-HIF2-alpha recombinant monoclonal [BL-95-1A2] (A700-003). Secondary: ReliaBLOT® reagents (WB120). Image Credit: Bethyl Laboratories Inc.
![Localization of human HIF1-alpha binding sites in immunoprecipitates from CoCl2 treated HepG2 lysates by ChIP-Seq. Antibody: Rabbit anti-HIF1-alpha recombinant monoclonal [BL-124-3F7] (A700-001).](https://www.news-medical.net/image-handler/picture/2021/3/art5-3.jpg)
Figure 3. Localization of human HIF1-alpha binding sites in immunoprecipitates from CoCl2 treated HepG2 lysates by ChIP-Seq. Antibody: Rabbit anti-HIF1-alpha recombinant monoclonal [BL-124-3F7] (A700-001). Image Credit: Bethyl Laboratories Inc.
HIF2A
In the United States, almost 64,000 cases of kidney cancers are diagnosed every year, and among these, 70% are categorized as clear-cell renal carcinoma (ccRCC). The von Hippel-Lindau tumor (VHL) suppressor gene is commonly mutated in ccRCC.
'Truncal' event refers to VHL loss in ccRCC: in other words, an early lesion that conspires with subsequent genetic hits to cause kidney cancer. HIF2-alpha builds up in the absence of VHL, causing the stimulation of transcriptional programs that accommodate angiogenesis and also the adaptation of cells to hypoxia.
A better interpretation of this mechanism has resulted in the discovery of several medications that specifically target HIF2-alpha effectors. For instance, the FDA has presently approved at least six medications that target VEGF or its receptor.
But in spite of the established efficacy of such medications, the clinical response to these kinds of agents has been found to be transitory because cancer cells can become resistant.
If HIF2-alpha could be directly targeted, a wider net could be cast to suppress the numerous signaling and transcriptional programs controlled by HIF2-alpha-responsive genes. Considering that transcription factors are regarded as undruggable, this approach has been assumed to be unapproachable.
But the discovery of a pocket in the PAS-B domain of HIF2-alpha that is responsible for controlling the dimerization between HIF2-alpha and ARNT - its obligatory partner - has promoted the concept of developing small molecule drugs that could inhibit dimerization, and therefore antagonize HIF2-alpha transcriptional activity.
Researchers from Peloton Therapeutics based in Dallas, Texas, have now identified a set of small molecular compounds that can antagonize HIF2-alpha transcriptional programs by suppressing the dimerization between HIF2-alpha and ARNT.
The anti-tumor efficacy of these compounds was assessed through in vitro analyses and also through studies involving xenograft ccRCC models at Peloton and in a couple of academic laboratories.
The research works demonstrated that one of these small-molecule drugs successfully suppressed the progression of tumors.
The most promising data also comes from the analysis by Chen and the team, which demonstrated how a patient suffering from metastatic ccRCC remained progression-free for a period of 11 months after being treated with the small-molecule drug.
In the following steps, predictive biomarkers need to be discovered to identify patients who would respond to therapies focused on dismantling the transcriptional programs of HIF2 and additional validation of HIF2-alpha as a target for treating ccRCC.
Bethyl’s Comparison
Immunocytochemistry (ICC) was used to evaluate the antibodies against HIF2-alpha for specificity. HIF2-alpha protein is stabilized under hypoxic conditions and quickly decomposed under normoxic conditions.
To activate the stabilization of HIF2-alpha, cobalt chloride treatment can be utilized as a mimetic agent for hypoxia. To compare the antibodies against HIF2-alpha, HepG2 cells treated with cobalt chloride or mock-treated were subsequently stained.
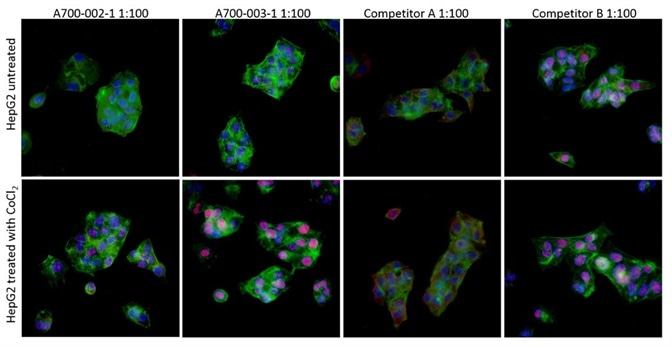
Figure 4. Image Credit: Bethyl Laboratories Inc.
The antibodies comprised a Bethyl recombinant rabbit monoclonal antibody (Catalog no A700-003), an extensively available mouse monoclonal, clone EP190b, from Competitor A, and also a goat polyclonal from Competitor B.
As predicted, in the case of untreated cells, there was no staining in any of the antibodies, except for the Competitor B goat polyclonal, which showed off-target nuclear staining.
In the case of treated cells, nuclear-localized, upregulated, HIF2-alpha was properly identified by Bethyl’s Cat# A700-003. This was unlike the antibody of Competitor A which identified an upregulated protein that was wrongly localized to the cytoplasm, and the antibody of Competitor B which did not change in response to the treatment.
*All antibodies were tested as per supplier datasheets.
![Detection of human HIF2-alpha (red) in formaldehyde-fixed asynchronous HepG2 cells untreated (left) and treated with CoCl2 (right) by ICC-IF. Antibody: Rabbit anti-HIF2-alpha recombinant monoclonal [BL-95-1A2] (A700-003). Secondary: DyLight® 594-conjugated goat anti-rabbit IgG (A120-201D4). Counterstain: DAPI (blue).](https://www.news-medical.net/image-handler/picture/2021/3/art5-5-1.jpg)
Figure 5. Detection of human HIF2-alpha (red) in formaldehyde-fixed asynchronous HepG2 cells untreated (left) and treated with CoCl2 (right) by ICC-IF. Antibody: Rabbit anti-HIF2-alpha recombinant monoclonal [BL-95-1A2] (A700-003). Secondary: DyLight® 594-conjugated goat anti-rabbit IgG (A120-201D4). Counterstain: DAPI (blue). Image Credit: Bethyl Laboratories Inc.
References
- Patel PH, Chadalavada RS, Chaganti RS, Motzer RJ. 2006. Targeting von Hippel-Lindau pathway in renal cell carcinoma. Clin Cancer Res. Dec 15;12(24):7215–20.
- Cho H, Kaelin WG. 2016. Targeting HIF2 in clear cell renal cell carcinoma. Cold Spring Harb Symp Quant Biol 81: 113–121.
- Wallace EM, Rizzi JP, Han G, Wehn PM, Cao Z, Du X, Cheng T, Czerwinski RM, Dixon DD, Goggin BS, et al. 2016. A small-molecule antagonist of HIF2α is efficacious in preclinical models of renal cell carcinoma. Cancer Res. Sep 15;76(18):5491–500.
- Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, et al. 2016. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. Nov 3;539(7627):112–117.
- Cho H, Du X, Rizzi JP, Liberzon E, Chakraborty AA, Gao W, Carvo I, Signoretti S, Bruick RK, Josey JA, et al. 2016. On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models. Nature. Nov 3;539(7627):107–111.
About Bethyl Laboratories, Inc.

Bethyl Laboratories, Inc. has been dedicated to improving lives by supporting scientific discovery through its qualified antibody products and custom polyclonal services since its founding in 1972. Bethyl has a global reputation for quality, consistency and first-class customer care. Every antibody that Bethyl sells is manufactured to exacting standards in Montgomery, Texas, and is validated in-house by a team of scientists. From the veterinary facilities to the development, production, and validation labs, the entire Bethyl team focuses on delivering quality products and delighting customers.
Bethyl Laboratories has been acquired by Fortis Life Sciences. To learn more visit: https://promotions.bethyl.com/news/fortis-life-sciences-acquires-bethyl-laboratories/.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.