Immune checkpoints have a vital role in controlling the duration and magnitude of an immune response. When immune checkpoints work as they should, a suitable response to insults like infection and malignancy is ensured, while harm to the host from too much immune reaction is also avoided.
Most significantly, immune checkpoint dysregulation caused by malignant cells can support their growth and expansion.1
The aim of anti-cancer immunotherapy is to reverse the aberrations and rather improve immune activity against malignant cells due to the potential of malignant cells to manipulate immune checkpoints.2
The field of immune checkpoint anti-cancer therapy has seen active drug development. Programmed cell death ligand-1 (PD-L1), programmed cell death receptor-1 (PD-1) and cytotoxic T lymphocyte-associated molecule-4 (CTLA-4) are the most extensively investigated checkpoints so far.3
Regrettably, the U.S. Food and Drug Administration-approved inhibitors targeting such immune checkpoints are therapeutically effective only in some patients suffering from cancer.
Even if a response is realized, it is common to develop resistance to these agents.4 Thus, there is a significant interest in devising innovative immune checkpoint therapies.
The use of TIGIT and its benefits
T cell immunoglobulin and ITIM domain (TIGIT) - also called VSIG9, Vstm3, and WUCAM - is one such target.5 Both natural killer (NK) cells and T cells express TIGIT.5
Although TIGIT expression tends to be weak on naive cells, it is induced quickly by antigenic challenge or inflammatory stimulus,5 involving high expression on tumor-infiltrating lymphocytes (TILs).6
TIGIT expression is linked to tumor progression, the release of immunoregulatory cytokines, direct immunosuppression of NK cells and T cell exhaustion.5,7,8
Preclinical studies have shown dual targeting of TIGIT and PD-1 to generate synergistic immune activation.9 The synergy might be at least partially described because when compared to CTLA-4, PD-L1, and PD-1, TIGIT suppresses immune responses induced by both NK cells and T cells.8
The differential expression and action of the several immune checkpoints point toward their independent, non-redundant functions.
The high expression of TIGIT on TILs but low expression in the periphery is another aspect that makes TIGIT an appealing target for immune checkpoint therapy.5 Therefore, targeting TIGIT can make the immune response work directly toward the target tumor while restricting systemic autoimmune reactions.
Better insights into the localization, mechanism of action, and role of TIGIT in the cancer immunity cycle will promote the advent of new anti-cancer immunotherapies. Moreover, further understanding of TIGIT’s function will enable the design of synergistic or complementary combination therapies.
TIGIT might be key to tackle the difficulties of treatment resistance, immune-associated toxicity, and restricted clinical utility of presently approved cancer immunotherapies. Although results of clinical trials of anti-TIGIT antibodies are yet to materialize, several trials are recruiting at present.10,11
The TIGIT antibodies offered by Bethyl are TIGIT Recombinant Monoclonal Antibody [BLR047F] (A700-047).
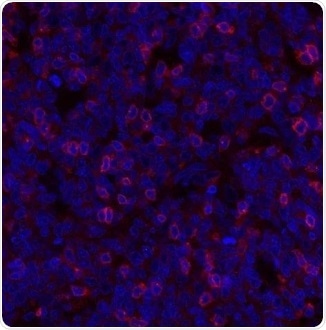
Figure 2. Detection of human TIGIT (red) in FFPE human tonsil. Antibody: Rabbit anti-TIGIT recombinant monoclonal [BLR047F] (A700-047). Secondary: Dylight 594 conjugated goat-anti-rabbit IgG (A120-101D4). Counterstain: DAPI (blue). Image Credit: Bethyl Laboratories Inc.
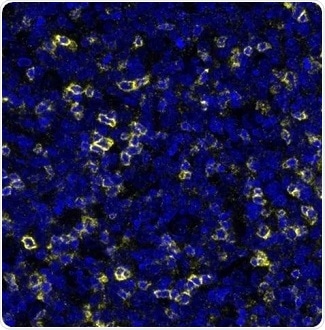
Figure 3. Detection of human TIGIT (yellow) in FFPE human tonsil. Antibody: Rabbit anti-TIGIT recombinant monoclonal [BLR047F] (A700-047). Secondary: HRP-conjugated goat-anti-rabbit IgG (A120-501P). Substrate: Opal. Counterstain: DAPI (blue). Image Credit: Bethyl Laboratories Inc.
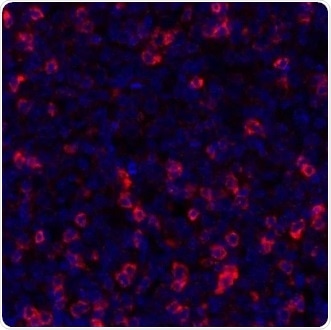
Figure 4. Detection of human TIGIT (red) in FFPE tonsil by IHC-IF. Antibody: Rabbit anti-TIGIT recombinant monoclonal [BLR047F] (A700-047). Secondary: HRP-conjugated goat anti-rabbit IgG (A120-501P). Substrate: Opal. Counterstain: DAPI (blue). Image Credit: Bethyl Laboratories Inc.
Conclusion
Image Credit: Bethyl Laboratories Inc.
Using B-cell sorting and recombinant DNA technology to its unmatched on-site manufacturing process, Bethyl delivers high-quality recombinant rabbit monoclonal antibodies (RmAbs).
These antibodies are designed with 100% assurance to work in validated applications to guarantee that users require less time to re-perform experiments and get more time to perform research.
References
- Pardoll DM. 2012. The blockade of immune checkpoints in cancer immunotherapy. Mar;12(4):252–64.
- Sharma P, Allison JP. 2015. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. Apr;161:205–14.
- Postow MA, Callahan MK, Wolchok JD. 2015. Immune checkpoint blockade in cancer therapy. J Clin Oncol. Jun;33(17):1974–82.
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. 2017. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. Feb;168:707–23.
- Manieri NA, Chiang EY, Grogan JL. 2017. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol. Jan;38(1):20–8.
- Chauvin JM, Pagliano O, Fourcade J, et al. 2015. TIGIT and PD-1 impair tumor antigen-specific CD8⁺ T cells in melanoma patients. J Clin Invest. May;125(5):2046–58.
- Marin-Acevedo JA, Dholaria B, Soyano AE, et al. 2018. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. Mar;11(1):39.
- Zhang Q, Bi J, Zheng X, et al. 2018. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. Jun;19:723–32.
- Anderson AC, Joller N, Kuchroo VK. 2016. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. May;44(5):989–1004.
- Clinicaltrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US). Accessed 2018 September 20. Available from: https://clinicaltrials.gov/ct2/show/NCT03119428.
- Clinicaltrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US). Accessed 2018 September 20. Available from: https://clinicaltrials.gov/ct2/show/NCT03563716.
About Bethyl Laboratories, Inc.

Bethyl Laboratories, Inc. has been dedicated to improving lives by supporting scientific discovery through its qualified antibody products and custom polyclonal services since its founding in 1972. Bethyl has a global reputation for quality, consistency and first-class customer care. Every antibody that Bethyl sells is manufactured to exacting standards in Montgomery, Texas, and is validated in-house by a team of scientists. From the veterinary facilities to the development, production, and validation labs, the entire Bethyl team focuses on delivering quality products and delighting customers.
Bethyl Laboratories has been acquired by Fortis Life Sciences. To learn more visit: https://promotions.bethyl.com/news/fortis-life-sciences-acquires-bethyl-laboratories/.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.