Sponsored Content by MaxCyte, Inc.Reviewed by Maria OsipovaMar 31 2023
The COVID-19 pandemic has caused widespread social and economic strain, leading to millions of fatalities worldwide. Testing is considered a crucial component of the public health response to combat this disease.
Continuous research efforts are necessary to understand the disease and develop effective interventions to address it.
The predominant technique employed to diagnose active COVID-19 infections is the detection of SARS-CoV-2 RNA through real-time reverse transcription PCR amplification. Antibody testing can also be used as a diagnostic approach.
Identifying antibodies the body produces in response to the SARS-CoV-2 virus is used to diagnose an infection. It usually takes approximately two weeks for a detectable level of antibodies to develop in the bloodstream.
Upon infection, the body rapidly produces specific antibodies (IgA and IgM), which are cleared quickly. On the other hand, other antibodies, such as IgG, take longer to be detected in the bloodstream. Identifying the presence of various isotypes of antibodies can assist in distinguishing between ongoing and previous infections.
Multiple antibody tests are available, each with its own set of advantages and limitations. Lateral flow immunoassays, for instance, are rapid and can provide results in under 20 minutes. However, these tests are not sensitive and provide only qualitative results.
Quantitative tests, such as enzyme-linked immunosorbent assays and chemiluminescent immunoassays, are more sensitive but time-consuming due to manual washing or complex fluidics steps.
MaxCyte Flow Electroporation® technology is aiding researchers in developing and implementing a fast, sensitive, and adaptable diagnostic test that addresses these limitations.
Researchers utilize biolayer interferometry (BLI) technology to measure the interference pattern of white light that reflects off an internal reference layer and a biosensor coated with immobilized proteins (antigens) in this innovative approach.
As antibodies bind to proteins on the biosensor, the optical density increases, leading to a shift in the reflected light's wavelength. This shift can be detected in real-time.
By using this real-time analysis, researchers can obtain accurate and precise information on binding specificity, antibody concentration, and avidity. The objective of this study was to evaluate whether this technology could detect SARS-CoV-2 antibodies.
Producing high-quality RBD proteins at scale is critical to the success of this diagnostic method. These proteins are used as antigens on the biosensor. MaxCyte® successfully produced high-grade antigens, enabling the quick and sensitive detection of SARS-CoV-2 antibodies in clinical samples.
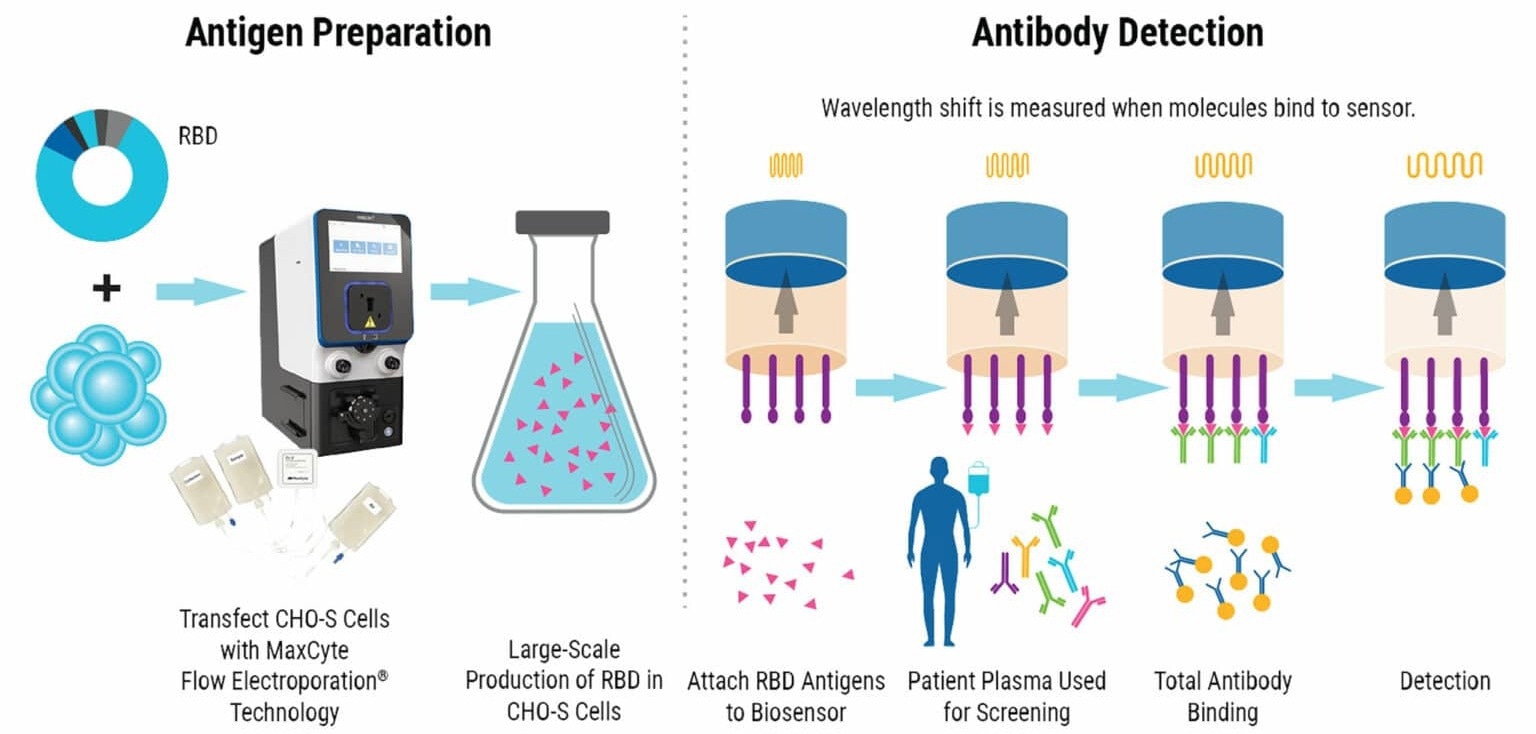
Image Credit: MaxCyte, Inc. adapted from Dzimianski et al. 2020 under Creative Commons License Attribution 4.0 International (CC BY 4.0)
Experimental design
MaxCyte® electroporation is used to transfect CHO-S cells with an expression plasmid for RBD, followed by harvesting and purifying the antigen from the cells. The biosensors are then dipped into RDB antigens and utilized to test plasma samples for the presence of antibodies against SARS-CoV-2.
Real-time measurements record the change in reflected light wavelength over time as antibodies bind to the RBD antigens, resulting in semi-quantitative detection of the antibodies present in patient plasma.
Aim
The study aimed to develop a BLI-ISA for detecting SARS-CoV-2 antibodies, and MaxCyte Flow Electroporation® technology was crucial in producing reliable RBD antigens for the biosensor.
The new diagnostic assay offers an automated process that can deliver real-time measurements of total and isotype-specific antibody levels in less than 20 minutes.
Method overview
The following is an outline of the RBD antigen production and the major steps involved in the BLI-ISA protocol, showcasing how MaxCyte Flow Electroporation® technology smoothly integrates into the simplified workflow for SARS-CoV-2 screening.
1. Plasmid Generation To create the expression plasmid for RBD, the cDNA encoding the protein was subcloned into a pcDNA3.1 derivative.
2. Antigen Production After transfecting CHO-S cells with the RBD expression plasmid via MaxCyte electroporation, the cells were incubated at 32 °C for eight days and subsequently centrifuged.
3. Protein Purification The StrepTrap® column eluted proteins, which were then biotinylated and dialyzed. Afterward, the proteins were purified again by using size exclusion chromatography. Finally, the purified proteins were flash-frozen at -80 °C for future use.
4. Biosensor Preparation BLI-ISA was carried out on an Octet® RED384
instrument using tilted bottom 384-well plates. Anti-biotin biosensors were
pre-equilibrated in buffer for 10 minutes before beginning the assay
5. Antigen Loading After equilibration, the biosensors were dipped into a solution of RBD antigen.
6. Total Antibody Binding The antigen-loaded sensors were dipped into a diluted patient serum sample, and the binding of antibodies was measured in real time by recording the change in the reflected wavelength over time. Wavelength shift was recorded after 10 minutes.
7. Detection The sensors were washed and then incubated with a secondary antibody (colloidal gold-conjugated anti-human IgG). Wavelength shift was recorded after three minutes.
Results
Total and specific antibody detection
To validate the BLI-ISA method, the researchers tested 10 plasma samples from recovered COVID-19 patients (which were presumed positive for antibodies) as well as 27 plasma and blood serum samples taken in 2016 and 2017 (before the COVID-19 pandemic).
In the validation of the BLI-ISA method, 37 samples (10 from recovered COVID-19 patients and 27 from before the pandemic) were tested at 1:8 dilution with antigen-coated sensors and no-antigen sensors.
Eight out of 10 positive samples were identified as positive by the Total Antibody Binding assay (as seen in Fig. 1). In the Detection step, all 10 positive samples showed a signal above the background. The trends were consistent with previous ELISA analyses of the same samples.
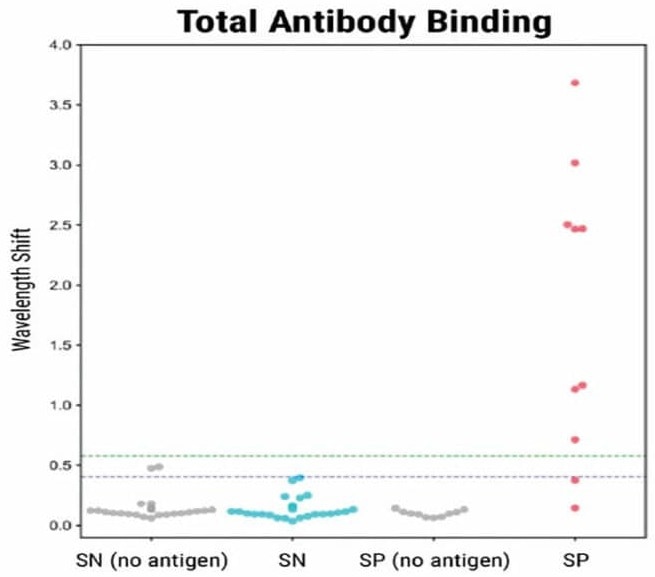
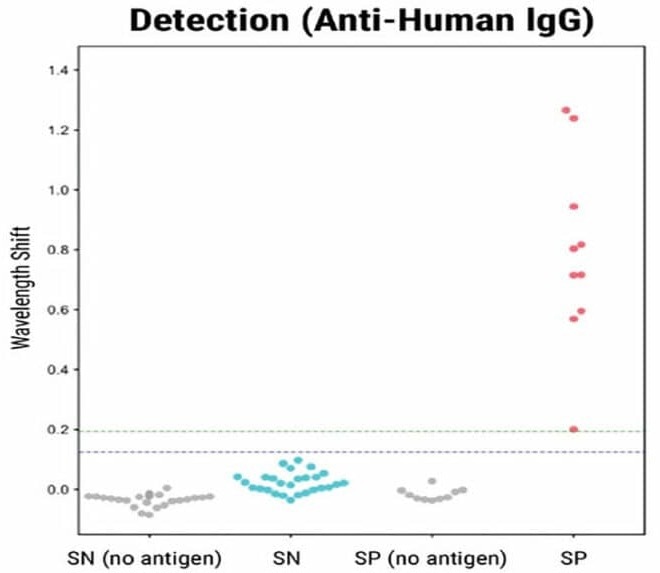
Figure 1. BLI-ISA evaluation of RBD-reactive human antibodies in human samples. BLI-ISA assay was used to measure SARS-CoV-2 specific antibodies from patient plasma. SN is seronegative, SP is seropositive. Assays were performed at 1:8 dilution. Dots represent the mean of biological duplicates for a given sample. Blue and green dashed lines represent the mean of all seronegative samples plus 3 and 5 standard deviations. Image Credit: MaxCyte, Inc. adapted from Dzimianski et al. 2020 under Creative Commons License Attribution 4.0 International (CC BY 4.0)
Specific SARS-CoV-2 detection
The specificity of the BLI-ISA method was assessed by testing the antigen binding of commercially obtained rabbit antibodies against the Spike proteins of both SARS-CoV-2 and HCoV-HKU1.
The positive control used in the specificity assessment was a human SARS-CoV-1 antibody known to cross-react with the SARS-CoV-2 spike RBD.
The HCoV-HKU1 antibodies did not cross-react with the SARS-CoV-2 antigen, whereas the SARS-CoV-2 antibodies showed a strong signal, as seen in Figure 2. The anti-human IgG secondary antibody used in the assay specifically binds to human antibodies but not rabbit antibodies.
The RBD-biotin antigen produced by MaxCyte® enabled precise identification and quantification of human antibodies against SARS-CoV-2.
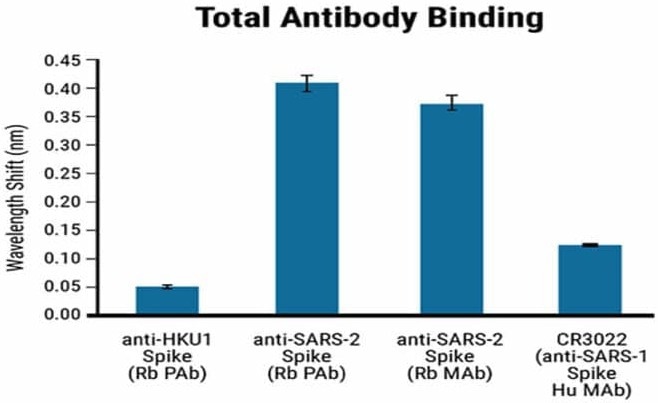
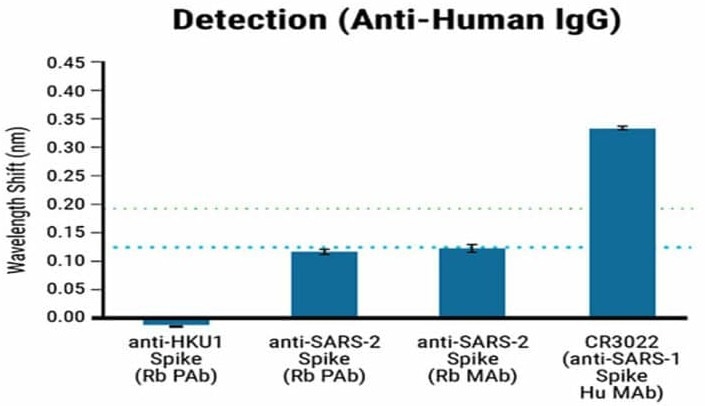
Figure 2. Specificity of the BLI-ISA method using Maxcyte® RDB antigen. BLI-ISA wavelength shift when RBD-biotin-loaded biosensors were dipped into Rabbit polyclonal antibody (Rb PAb), anti-HKU1 Spike (1.5 µg/mL), Rb PAb anti-SARS-CoV-2 Spike (1.5 µg/mL), rabbit monoclonal antibody (Rb MAb) anti-SARS-CoV-2 Spike (0.335 µg/mL), or human monoclonal antibody (Hu MAb) anti-SARS-CoV-1 Spike CR3022 (0.2 µg/mL). Error bars represent one standard deviation. Bars represent the mean of biological duplicates. Blue and green dashed lines represent the mean of all seronegative samples plus 3 and 5 standard deviations. Image Credit: MaxCyte, Inc. adapted from Dzimianski et al. 2020 under Creative Commons License Attribution 4.0 International (CC BY 4.0)
Quantitative assessment of antibody levels
Dilution series experiments demonstrated a clear correlation between sample concentration and signal strength for Total Antibody Binding, as shown in Figure 3.
The BLI-ISA method detected relative total SARS-CoV-2 antibody levels in moderately and strongly positive samples and qualitatively confirm IgG antibodies at a 1:8 dilution.
Samples with ambiguous or weak positive results can be re-evaluated at a higher concentration. For a quantitative assessment of IgG levels, dilution beyond 1:8 may be necessary due to the saturation effect on the biosensor at high concentrations.
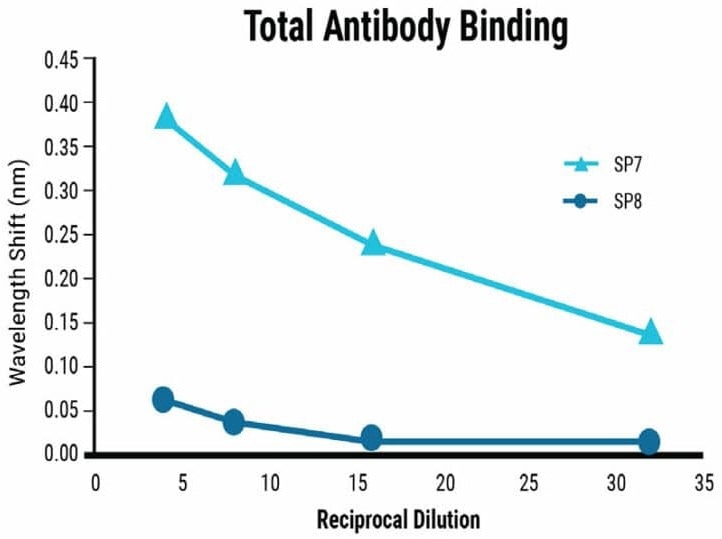
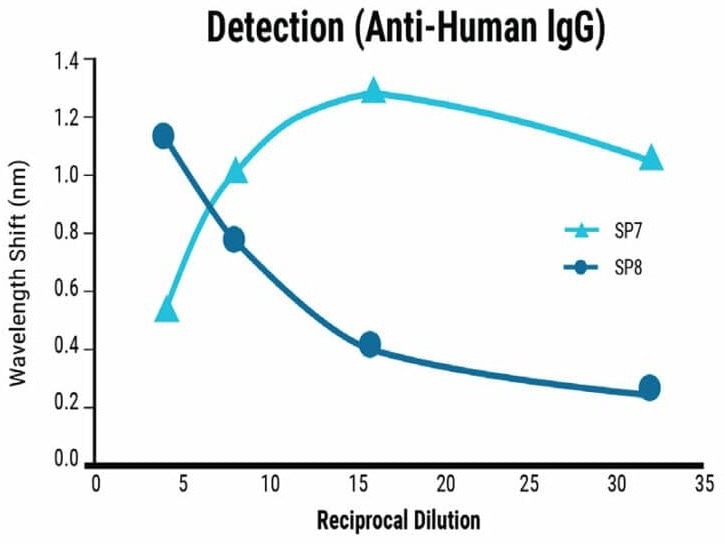
Figure 3. Sensitivity of the BLI-ISA method. Dilution series from strongly (SP7) and moderately (SP8) seropositive samples were tested using the BLI-ISA method to determine assay sensitivity. Detection with anti-human IgG secondary determined the strength of the antibody response in each sample. Image Credit: MaxCyte, Inc. adapted from Dzimianski et al. 2020 under Creative Commons License Attribution 4.0 International (CC BY 4.0)
Adaptable for different antibodies and antigens
The BLI-ISA method was also tested using a prefusion-stabilized Spike trimer as the antigen. Although the overall signal was lower compared to the RBD antigen, the measurement trends were consistent.
The SP samples showed a negative wavelength shift during the Detection step, which was attributed to the increased size of the prefusion spike compared to RBD.
The researchers found that adapting the Detection step to quantify IgA instead of IgG showed lower signal strength, likely due to the lower prevalence of IgA in the blood.
However, the method still showed moderate success in detecting IgA (as shown in Figure 4). These results show that the BLI-ISA method can be modified and adapted to detect other antigens and antibodies beyond SARS-CoV-2.
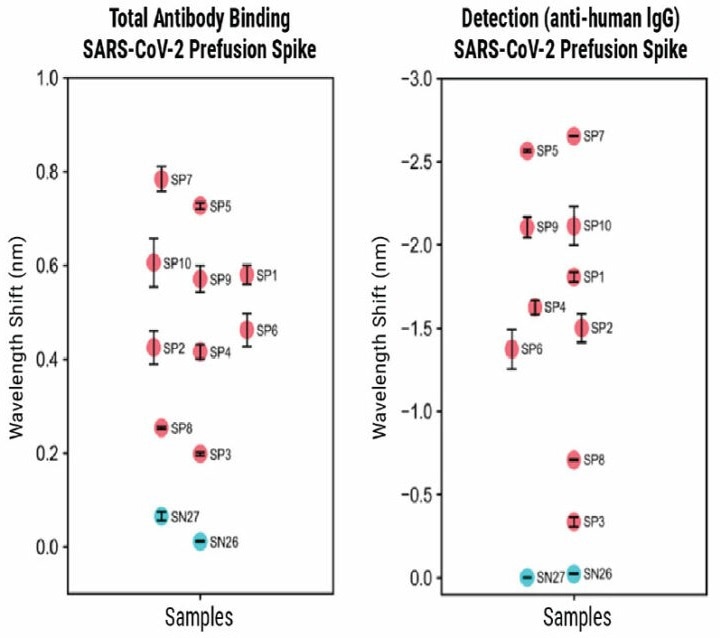
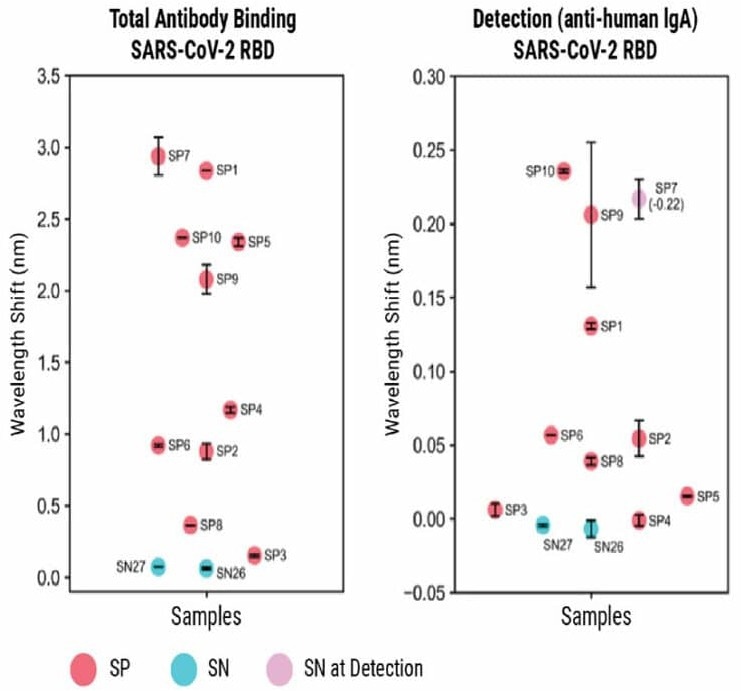
Figure 4. BLI-ISA evaluation of plasma IgG to SARS-CoV-2 prefusion spike and plasma IgA to SARS-CoV-2 spike RBD. BLI-ISA results for selected samples using the SARS-CoV-2 prefusion spike antigen for Total Antibody Binding and IgG Detection (top) or SARS-CoV-2 spike RBD for Total Antibody Binding and IgA Detection (bottom). SN is seronegative, SP is seropositive. Image Credit: MaxCyte, Inc. adapted from Dzimianski et al. 2020 under Creative Commons License Attribution 4.0 International (CC BY 4.0)
Conclusion and future applications
The use of MaxCyte Flow Electroporation® technology facilitated the production of large amounts of SARS-CoV-2 antigen, which was necessary for developing the BLI-ISA method for measuring antibodies against the virus.
The technology enabled efficient transfection of CHO-S cells with the RBD expression plasmid, resulting in high levels of antigen production. The BLI-ISA is an adaptable and simple procedure that has the potential for scalable high-throughput screening and can be used to detect various antigen and antibody isotypes.
This study demonstrates that MaxCyte's ExPERTTM technology is scalable and can support the development of new diagnostic assays, such as the BLI-ISA method, for detecting SARS-CoV-2 antibodies.
BLI-ISA provides fast antibody data to study infection prevalence, immunity duration, interventions, and vaccine development.
References
Dzimianski JV, Lorig-Roach N, et al. Rapid and sensitive detection of SARS-CoV-2 antibodies by biolayer interferometry. Scientific Reports. 2020;10(1). Published 2020 Dec 10 doi:10.1038/s41598-020-78895-x
About MaxCyte, Inc.
MaxCyte is a leading commercial cell-engineering company focused on providing enabling platform technologies to advance innovative cell-based research as well as next-generation cell therapeutic discovery, development and commercialization. Over the past 20 years, we have developed and commercialized our proprietary Flow Electroporation® platform, which facilitates complex engineering of a wide variety of cells.
Our ExPERT™ platform, which is based on our Flow Electroporation technology, has been designed to support the rapidly expanding cell therapy market and can be utilized across the continuum of the high-growth cell therapy sector, from discovery and development through commercialization of next-generation, cell-based medicines. The ExPERT family of products includes: four instruments, the ATx™, STx™, GTx™, and VLx™; a portfolio of proprietary related processing assemblies or disposables; and software protocols, all supported by a robust worldwide intellectual property portfolio.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.