Sponsored Content by MaxCyte, Inc.Reviewed by Maria OsipovaApr 13 2023
Efficient and reliable delivery of DNA, mRNA, and Ribonucleoprotein (RNP)-based reagents into primary and stem cells is imperative for precision genome engineering.
For clinical gene editing, a transfection platform that does not compromise genome stability and cell viability, is required. The CRISPR-Cas9 system presents promising potential for treating various genetic disorders.
Nonetheless, unintended off-target double-stranded breaks resulting from Cas9 cleavage may lead to unforeseeable clinical complications.
The present study characterizes genome integrity in clinically relevant human hematopoietic stem and progenitor cells (HSPCs) after CRISPR-Cas9 transfection utilizing the MaxCyte® electroporation technology to address this issue.
The findings indicate no substantial structural or nucleotide sequence alterations within the genomes of clonal isolates obtained from human HSPCs following electroporation and Cas9 treatment.
Experimental design
Two Cas9 RNPs engineered to target two distinct loci, CXCR4 and AAVS1, were electroporated into primary human hematopoietic stem and progenitor cells (HSPCs). Gene-edited HSPCs and controls were then subjected to single-cell cloning in 96-well plates.
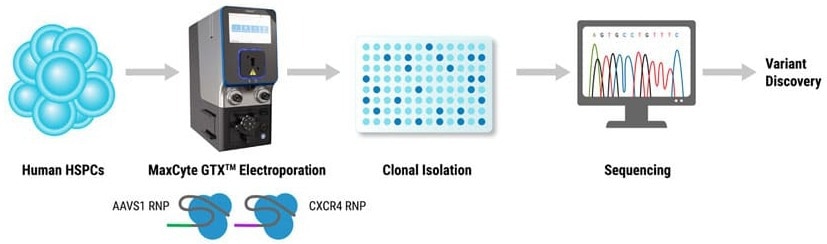
Image Credit: MaxCyte, Inc.
To assess genomic integrity and Cas9 activity, whole-genome sequencing (WGS) and bioinformatics analysis were performed in an unbiased manner.
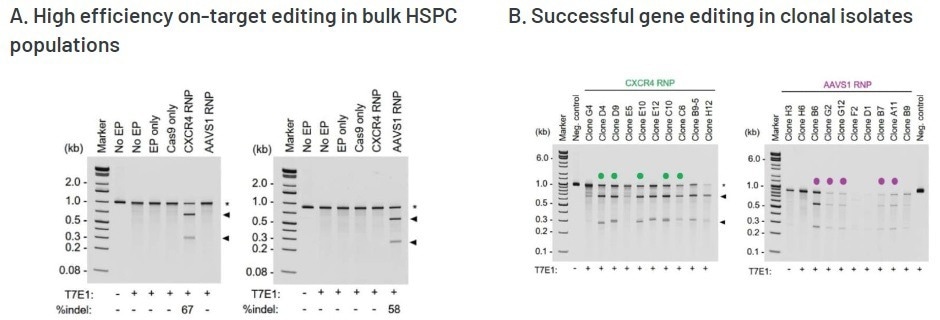
Editing efficiency was evaluated in bulk (pre-clonal) HSPCs and cellular clones using a T7E1 assay. The CXCR4 and AAVS1 on-target InDel formation rates in bulk HSPCs were 67% (left) and 58% (right), respectively. The gene editing efficiencies were deemed high, considering the accepted limits of sensitivity of the T7E1 assay.
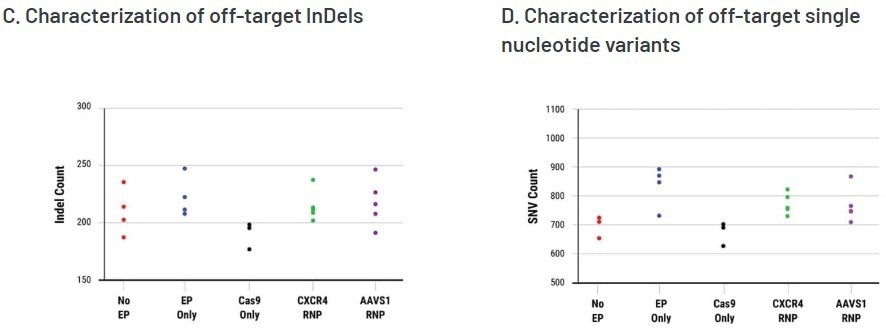
The WGS analysis revealed a total of 4,474 off-target InDels and 15,855 off-target single nucleotide variants (SNVs) distributed among all the samples. The majority of InDels and SNVs were observed in intergenic or non-protein coding regions of the genome.
Regardless of the presence of Cas9 RNPs, both electroporated cells and untreated cells displayed similar distributions of both InDels and SNVs.
Genome-wide sequencing analysis of CRISPR-Cas9 activity in human HSPCs.
Editing efficiency was determined in A) bulk (pre-clonal) HSPC and B) cellular clones using a T7E1 assay. On-target InDel formation in bulk HSPCs was 67% for CXCR4 (left) and 58% AAVS1 (right). Gene editing efficiencies were high, given the accepted limits of sensitivity of the T7E1 assay. Clonal isolates selected for WGS analysis are indicated by a color-coded dot. No EP=non-electroporated cells; EP Only=Cells electroporated without CRISPR RNP; Cas9 Only=Cells electroporated without gRNA. WGS revealed a total of C) 4,474 off-target InDels and D) 15,855 off-target single nucleotide variants (SNVs) distributed among all the samples. The majority of InDels and SNVs appeared within intergenic or non-protein coding regions within the genome. Electroporated cells and untreated cells displayed similar distributions of both InDels and SNVs regardless of the presence of Cas9 RNPs. Image Credit: MaxCyte, Inc., adapted from Smith et al. 2020 under Creative Commons License Attribution CC BY 4.0.
Summary
- MaxCyte® electroporation's high efficiency and low toxicity provide highly focused gene disruption frequencies with minimum off-target effects, enhancing therapeutic effectiveness.
- Gene editing using MaxCyte non-viral engineering facilitates creation of next-generation adoptive cell treatments for a broad range of disorders.
- MaxCyte electroporation effectively delivers a variety of payloads, such as mRNA, sgRNA, and RNPs, to difficult-to-engineer primary cells such as hematopoietic stem cells and T cells utilized in adoptive cell treatments.
- MaxCyte electroporation enables high levels of gene editing including:
- Gene knockout/disruption
- Gene knock-in
- Single nucleotide gene mutation correction
- MaxCyte electroporation of new cell types and payloads is a quick and simple procedure.
References
Smith R, Chen Y, Seifuddin F, et al. Genome-Wide Analysis of Off-Target CRISPR-Cas9 Activity in Single-Cell-Derived Human Hematopoietic Stem and Progenitor Cell Clones. Genes. 2020;11(12):1501. Published 2020 Mar 13. doi :10.3390/genes112150
About MaxCyte, Inc.
MaxCyte is a leading commercial cell-engineering company focused on providing enabling platform technologies to advance innovative cell-based research as well as next-generation cell therapeutic discovery, development and commercialization. Over the past 20 years, we have developed and commercialized our proprietary Flow Electroporation® platform, which facilitates complex engineering of a wide variety of cells.
Our ExPERT™ platform, which is based on our Flow Electroporation technology, has been designed to support the rapidly expanding cell therapy market and can be utilized across the continuum of the high-growth cell therapy sector, from discovery and development through commercialization of next-generation, cell-based medicines. The ExPERT family of products includes: four instruments, the ATx™, STx™, GTx™, and VLx™; a portfolio of proprietary related processing assemblies or disposables; and software protocols, all supported by a robust worldwide intellectual property portfolio.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.