Recent advancements in next-generation sequencing have led to substantial increases in throughput, coupled with reduced expenses.
Due to these improvements, the process of library preparation has become even more of a financial and time constraint to high throughput sequencing core facilities. Nonetheless, with the help of automation in the form of liquid-handling robots, some of these bottlenecks can be alleviated.
This article outlines the utilization of one such system, the mosquito® HV system, to efficiently execute DNA library preparation for Illumina sequencing, reducing the volume to one-tenth of the original manual protocol.
Key benefits
mosquito HV genomics, a fully open liquid handler, offers several advantages:
- Low-cost DNA library preparation achieved by reducing reagent volumes
- Improved reproducibility and data quality via accurate pipetting, regardless of liquid viscosity
- Enables swift and high-throughput microbiome studies
The mosquito system, developed by SPT Labtech, employs positive displacement for pipetting, utilizing disposable tips containing a small stainless steel rod (see Figure 1).
This pipetting mechanism allows for precise handling of nanoliter volumes, eliminating the need for specialized liquid classifications in the instrument control software.
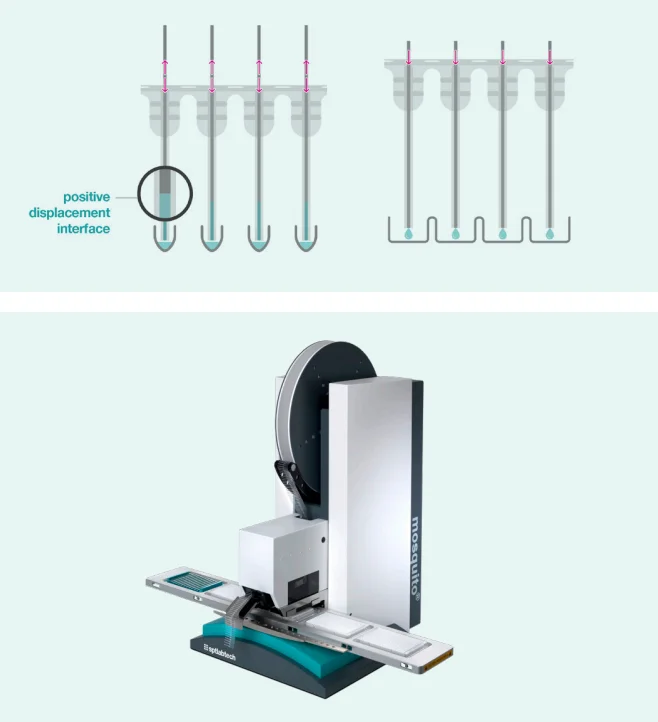
Figure 1. mosquito positive displacement tip technology and mosquito HV genomics. Image Credit: SPT Labtech
The selection of the NEBNext Ultra II FS DNA method was based on its enzymatic fragmentation capability, eliminating the need for physical DNA fragmentation at the onset of library preparation.
Using ZymoBIOMICS Microbial Community DNA Standard as genomic DNA, the performance of the mosquito HV system was compared with manually prepared libraries at standard volumes, using varying DNA input quantities.
To evaluate outcomes with less standardized input material, reduced-volume NEBNext Ultra II FS DNA libraries were generated from individual bacterial isolates and compared with manually prepared Illumina TruSeq Nano libraries derived from the same input material.
Additionally, a comparison was made between the NEBNext Ultra II FS DNA automated libraries and TruSeq Nano and TruSeq PCR Free libraries produced through manual methods or traditional automation.
Manual Vs. automated
To assess the performance of the NEBNext Ultra II FS DNA library kit, the ZymoBIOMICS Microbial Community DNA Standard (Cat. No. D6306) was utilized.
This standard contains a known DNA composition of 10 microbial strains, enabling the evaluation of entire metagenomic workflows. It highlights potential biases and errors that may arise during processing, making it an ideal tool for testing library preparation kits.
It allows for a direct performance comparison with previously tested library kits at the CGR. Testing was conducted in triplicate using total input amounts of 50 ng, 1 ng, and 0.1 ng, as illustrated in Figure 2.
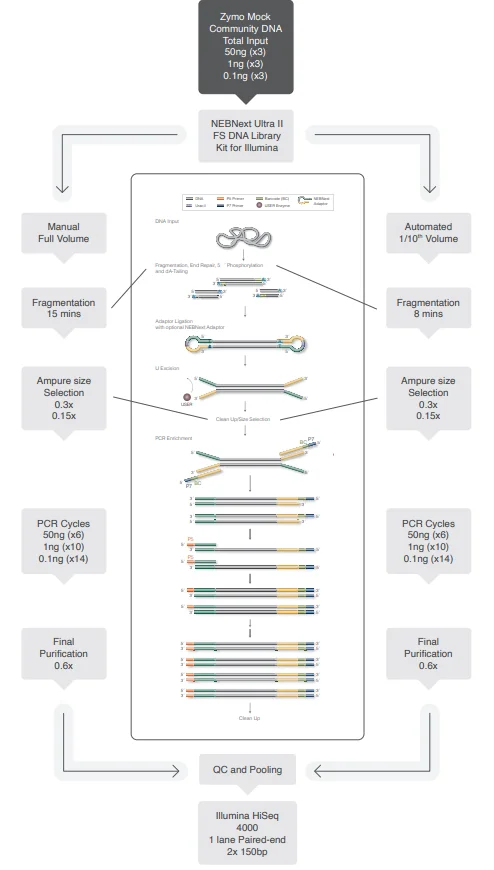
Figure 2. Schematic representation detailing how NEBNext libraries were generated using the ZymoBIOMICS Microbial Community DNA Standard as input DNA. Manual libraries were made using full volumes according to protocol, automated libraries were made using 1 in 10 volume on the SPT Labtech mosquito HV platform. Image Credit: SPT Labtech
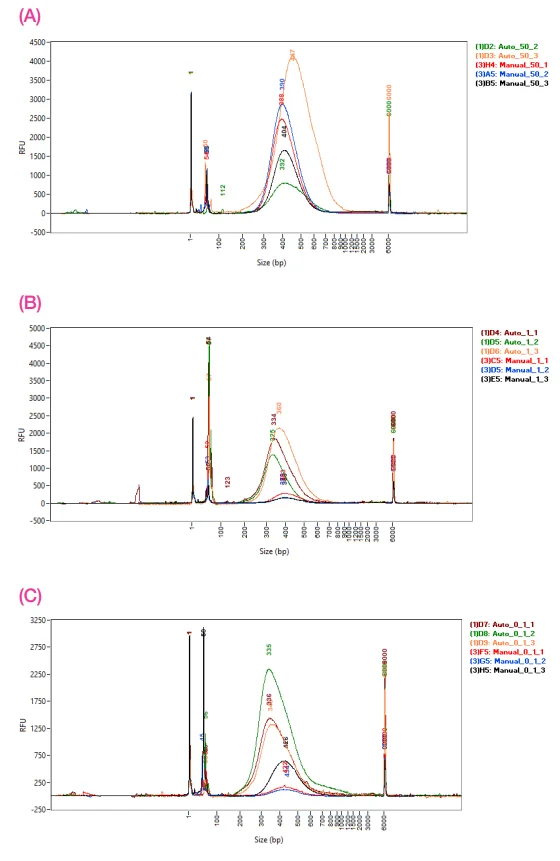
Figure 3. Fragment Analyzer traces comparing the size distribution of manual and 1/10 automated libraries for A) 50 ng, B) 1 ng, and C) 0.1 ng of input DNA. Image Credit: SPT Labtech
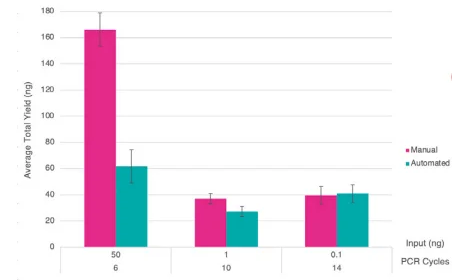
Figure 4. Bar chart showing average yields for the manual and 1/10 automated libraries with differing input amounts and PCR cycle numbers. Error bars indicate standard deviation. Image Credit: SPT Labtech
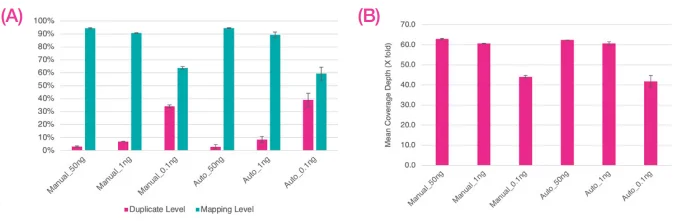
Figure 5. Sequence data were normalized to 13M reads per library, A) indicates average levels of duplicate and mapping percentages, B) shows average fold coverage. Error bars indicate standard deviation. Image Credit: SPT Labtech
Comparative performance on clinical and bacterial isolates
Although the ZymoBIOMICS Microbial Community DNA Standard yielded promising results, it is not always indicative of performance with real-world samples.
Previously generated data from clinical bacterial isolates utilized manually prepared TruSeq Nano libraries with 100 ng of input DNA. The same samples were subjected to the automated 1 in 10 volume NEBNext Ultra II FS DNA method with 10 ng of input DNA, and the results were compared.
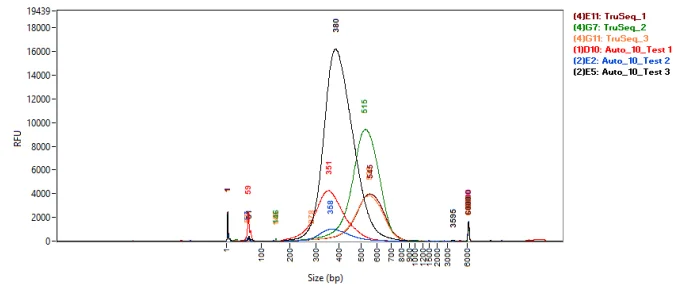
Figure 6. Fragment Analyzer traces comparing the size distribution of manual TruSeq Nano and 1/10 volume automated NEBNext Ultra II FS DNA libraries. Image Credit: SPT Labtech
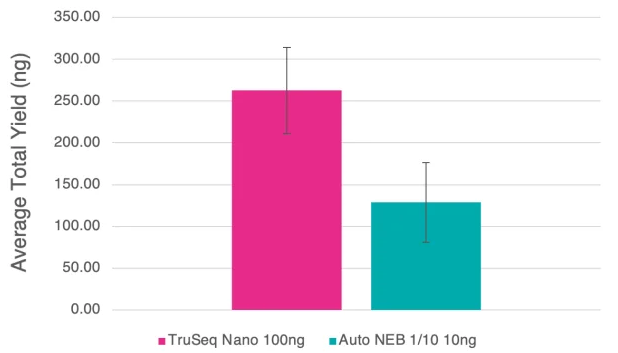
Figure 7. Bar chart detailing average yields for the manual TruSeq Nano and 1/10 automated NEB libraries generated from clinical bacterial isolates. 100 ng and 10 ng of input DNA were used for the TruSeq Nano and NEB 1/10 libraries, and 8 and 10 cycles of PCR, respectively. Error bars are standard deviation. Image Credit: SPT Labtech
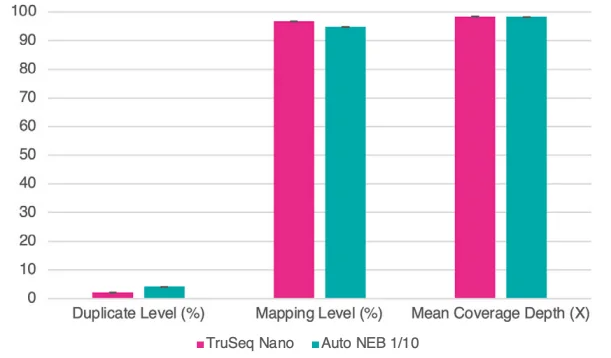
Figure 8. Sequence data were normalized to 1.3M reads per library. Bar chart shows averages of duplicate and mapping percentages, and fold coverage of the genome. Error bars are standard deviation. Image Credit: SPT Labtech
Comparison of automated 1/10 volume NEB Ultra II FS libraries with full volume automated and manual TruSeq Nano and TruSeq PCR Free libraries
Previously, workflows in the CGR used Illumina TruSeq Nano or TruSeq PCR Free libraries. Data was generated using these methods, both manually and with Beckman full volume automation on the FXP system.
Input included 100 ng and 1 µg of the ZymoBIOMICS Microbial Community DNA Standard for TruSeq Nano and TruSeq PCR Free libraries, respectively. Data demonstrated comparability across all three methods when 50 ng of DNA was employed as input for the 1 in 10 volume NEBNext Ultra II FS DNA libraries.
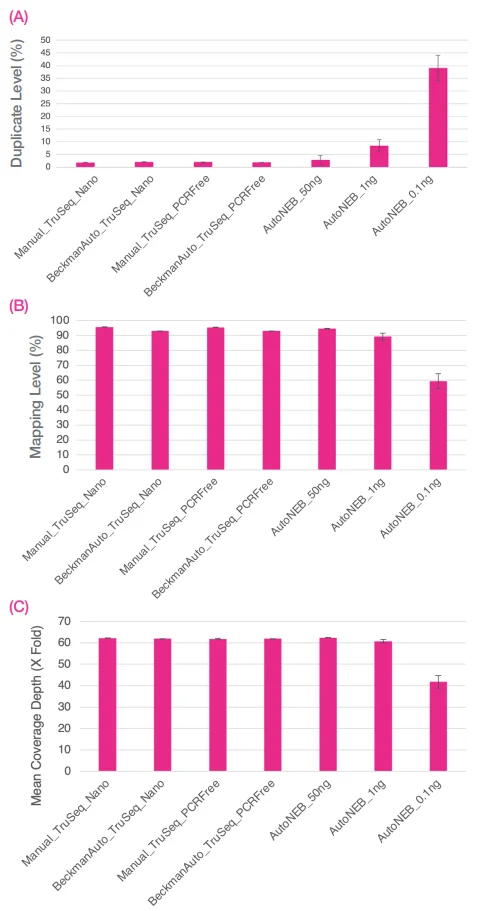
Figure 9. Sequence data were normalized to 13M reads per library. Bar chart shows averages of A) percentage duplicates, B) percentage mapping, and C) fold coverage (X) of the mock community. Error bars are standard deviation. Image Credit: SPT Labtech
Conclusion
This article demonstrates that NEBNext Ultra II FS DNA libraries prepared at 1 in 10 performed as well as the full volume manually prepared libraries while providing significant cost savings through the miniaturization of reaction volumes.
Even with input DNA as low as 1 ng, comparable percentages of duplicates, mapping levels, fold coverage, and GC skew (not shown here) were observed. The entire workflow can be completed in a single day, facilitating larger projects with more samples and reduced technical bias.
About SPT Labtech
SPT Labtech designs and manufactures robust, reliable and easy-to-use solutions for life science. We enable life scientists through collaboration, deep application knowledge, and leading engineering to accelerate research and make a difference together. We offer a portfolio of products within sample management, liquid handling, and multiplexed detection that minimize assay volumes, reduce material handling costs and put the discovery tools back in the hands of the scientist.
At the Heart of What We Do
Many of our innovations have been born out of the desire to create solutions to existing customer problems; and it’s this ethos that drives SPT Labtech’s R&D efforts. Our strengths come from the trust our customers have with us to develop truly unique, automated technologies to meet their needs. We combine cutting edge science with first-rate engineering to put customers at the heart of everything we do.
A Problem-Solving State of Mind
The substantial breadth of expertise within our company enables us to be involved in the full life cycle of our products from the initial design concept, mechanical and software engineering and prototyping, to final manufacture and sale. These qualities allow us to offer the best possible technical and mechanical support to all the equipment that we supply, hence maintaining excellent client relationships.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.