L.B. Bohle has been a technological leader in all film coating applications for many years. L.B. Bohle Coaters guarantees good product processing at the highest profitability. It asserts its leadership position through continuous development and patented advantages.
Pharmaceutical film coating is currently an important production step in the pharmaceutical business. Coating technology has evolved over the last few decades, beginning with dragee pan processing.

Image Credit: Roysstock/Shutterstock.com
The bulk of coating procedures are used to modify drug release, increase drug stability against light and moisture, and hide flavor. In addition, patient compliance issues are crucial, such as improving swallowability or making identification easier due to a different color.
API coating is becoming increasingly important since it allows for fixed-dose combinations or the mixing of incompatible medications. Sustained-release coatings, in addition to rapid-release coating layers, can achieve different medication-release properties.
Such formulations can contain up to four coating layers, resulting in extended processing periods. Coating uniformity is a necessity and a quality trait for effectively developing and producing such formulations, as coated tablets must pass the pharmacopeias' dosage unit uniformity test.1,2
Because a coating process involves simultaneous spraying, mixing, and drying, selecting the appropriate parameters is essential for achieving high coating uniformity. In recent years, L.B. Bohle has created and enhanced its coater design to suit these needs.
The L.B. Bohle Coating Technology incorporates three distinct design elements that ensure optimum coating uniformity.
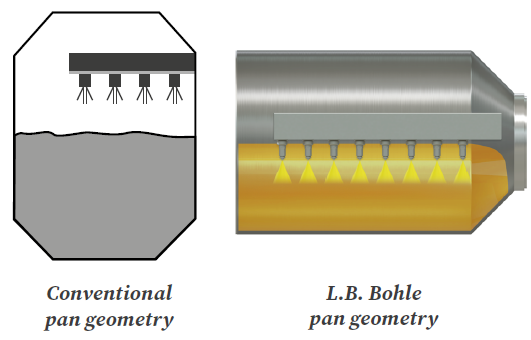
Figure 1. Pan geometry. Image Credit: L.B. Bohle Maschinen und Verfahren GmbH
L.B. Bohle pan geometry with an L/D ratio >1 provides a broad tablet bed area, allowing for the use of a large number of spray guns (Figure 1).
Due to increased coating suspension throughput, process time can be reduced by up to 40% compared to typical pan geometries on the market. Furthermore, the smaller tablet bed causes minimal shear on the tablets, allowing the coating of even the weakest tablets.
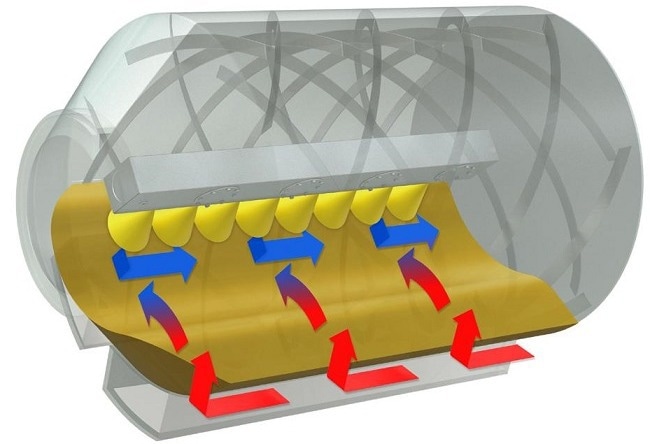
Figure 2. Helicle baffles. Image Credit: L.B. Bohle Maschinen und Verfahren GmbH
The distinctive helical baffles are made up of two layers of baffles (Figure 2). They are in charge of ensuring constant and homogenous axial mixing within the tablet bed. In addition, the drum rotation maintains the radial mixing. Both actions ensure a dead zone-free tablet bed.
Thus, a homogenous mixture in the tablet bed is typically established after a few minutes. Because of the continual tablet movement, no acceleration peaks occur, which could cause tablet damage or even twinning.
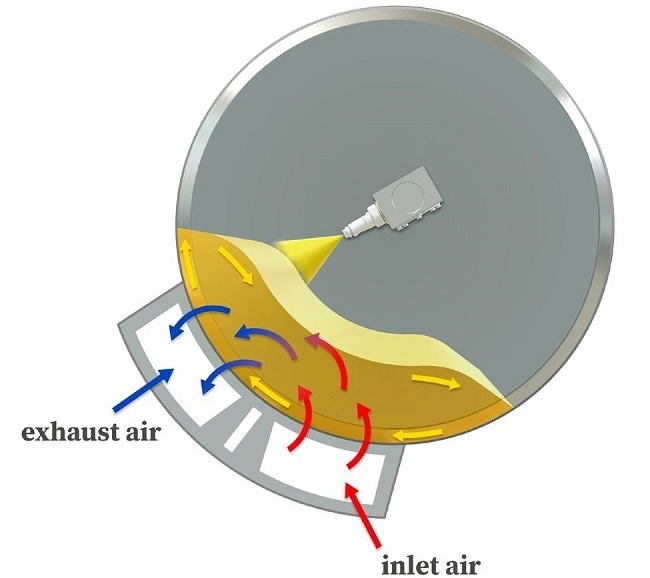
Figure 3. Air flow principle. Image Credit: L.B. Bohle Maschinen und Verfahren GmbH
The air principle of L.B. Bohle Film Coaters directs drying capability to where it is most needed: the tablet bed (Figure 3). As a result, the drying capacity of the incoming air is maximized while the remaining coater interior parts are not heated.
The inlet air enters the exhaust air funnel straight via the spinning tablet bed coming from beneath it. This configuration also has another advantage: The spray guns are not heated while coating and remain cool.
As a result, spray losses are minimized, resulting in coating efficiencies of greater than 95%, which is particularly useful for API coating operations. Aside from the unique qualities that have contributed to the success of L.B. Bohle Coating Technology, these coating equipment can be equipped with CIP systems.
At high pressures, a cleaning lance is used to properly clean the coater after manufacturing in an automated mode.
L.B. Bohle coating equipment is offered in various sizes, from experimental to big production scale. Furthermore, multiple configuration lines are accessible throughout all continents to fulfill customers' diverse needs. In addition to the traditional batch mode machine types, semi-continuous coaters are also available.
References and further reading
- European Pharmacopoeia, Monograph 2.9.40, Uniformity of Dosage Units, 7th Edition, Directorate for the Quality of Medicines of the Council of Europe, Strasbourg, 2011.
- United States Pharmacopoeia, Monograph Uniformity of Dosage Units, 35th edition, US Pharmacopoeial Convention, Rockville, 2011.
- Optimization of inter-tablet coating homogeneity in an active coating process, Just S. et al., Poster presentation at the AAPS Annual Meeting, Chicago 2012.
About L.B. Bohle Maschinen und Verfahren GmbH
L.B. Bohle with the headquarter in Germany, is one of the largest system suppliers for the pharmaceutical processing industry and related sectors. Internationally active, they focus on machinery and equipment as well as process technology and components.
In addition, L.B. Bohle offer sustainable solutions for demanding production processes in batch and continuous manufacturing for the oral solid dosage production. For instance, L.B. Bohle provides single or interlinked machines for the processes Weighing, Dry and Wet Granulation, Grinding and Sieving, Container Blending, Tablet Coating and Tablet Handling.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.