Cardiotoxicity underlies a third of failures in regulatory clearance, making it among the most significant hurdles in developing new drugs.1 axoCells human iPSC-derived ventricular cardiomyocytes have been built as a robust model for cardiotoxicity testing that is relevant to humans.
Cardiac safety pharmacology has been met with well-defined challenges, such as fatal arrhythmias like Torsades de Pointes (TdP) that underlie 14 major drug withdrawals.2, 3 To assess this, various cardiotoxicity models have been utilized, such as in vivo (animal) models, primary cell models (cells collected directly from humans or animals), and in vitro immortalized cells transfected with ion channels. Although such models have some value, a translational hurdle remains in transferring results from the lab to the clinic, underscoring the need for more model systems more capable of translating results to humans.
In vivo cardiotoxicity models reportedly provide only selected insights into potential drug interactions.4 In vitro models developed from transfecting cell lines with a single ion channel gene also provide limited insights, as they may overlook other critical effects of ion channels that might make up for those single ion channel effects.1, 3
An advancement in in vitro single-channel models might be seen in “multichannel” models, more representative of the complex ion channel behavior underlying the human heart’s action potentials. These models can convey the characteristic initial depolarization largely owing to early sodium channels, plateau phase from a balance of calcium influx and potassium efflux, and rapid repolarization from potassium channels (especially the hERG channel) with hERG blockade resulting in prolongation of action potential duration (APD) and arrhythmia. Repeating this interaction is crucial for developing physiologically-relevant models.
The significance of a multi-channel model is most aptly demonstrated via the therapeutic verapamil, a commonly used compound that results in hERG blockade and thus would not pass a single-channel hERG block model. Yet, multi-channel effects (mostly on calcium channels) compensate for the prolongation of APD, averting the occurrence of arrhythmia.
| |
High TdP risk |
Intermediate TdP risk |
Low TdP risk |
| Training set |
Bepridil
Dofetilide
Quinidine
Sotalol |
Chlorpromazine
Cisapride
Terfenadine
Ondansetron |
Diltiazem
Mexiletine
Ranolazine
Verapamil |
| Validation |
Azimilide
Ibutilide
Vandetanib
Disopyramide |
Astemizole
Clarithromycin
Clozapine
Domperidone
Droperidol
Pimozide
Risperidone |
Loratidine
Metoprolol
Nifedipine
Nitrendipine
Tamoxifen |
Figure 1. The twenty eight compounds that form the panel for CiPA testing of cardiomyocytes. Image Credit: Axol Bioscience Ltd
Materials and methods
axoCells iPSC-derived ventricular cardiomyocytes (ax2508) were sent to Clyde Biosciences for an external evaluation of the cell's response to the 28 CiPA compounds via their CellOPTIQ® platform system. Cells were grown for six days, then incubated with voltage-sensitive dyes (VSD) in serum-free media, and lastly, treated with compounds at four different concentrations (n=6). Vehicle/negative control was DMSO at just 0.1 %. Meanwhile, positive control was Dofetilide at 3nM.
It is thus essential to assess compounds using more relevant model systems. The Comprehensive Pro-Arrythmia Assay (CiPA) initiative was built as a more precise means of testing the pro-arrhythmic capacity of novel drugs and utilizes multi-channel readings. The assay utilizes twenty-eight therapeutics of previously outlined TdP risk, highlighting in the following table.
Results
External validation yielded a predictable outcome for all 28 compounds in the CiPA panel. Responses were noted at various concentrations for all compounds and evaluated for signs of arrhythmia and quiescence (represented as ‘Q’ on the traces).
Information for all 28 compounds is obtainable upon inquiry, and the outcomes from three crucial compounds are displayed below:.
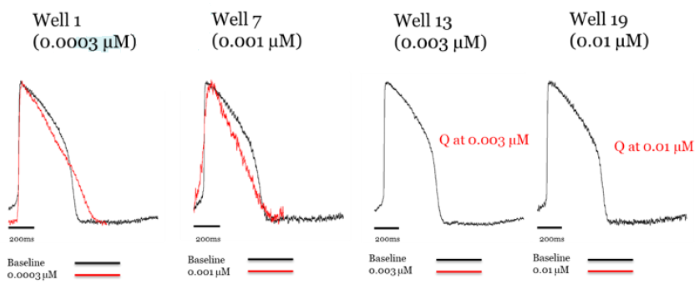
Figure 2. Addition of classic hERG blocker dofetilide to axoCells ventricular cardiomyocytes produces hERG blockade at low concentrations (demonstrated by triangulation of the action potential ), with quiescence at high concentrations. Raw trace comparing control (black) to treatment with dofetilide (red). Image Credit: Axol Bioscience Ltd
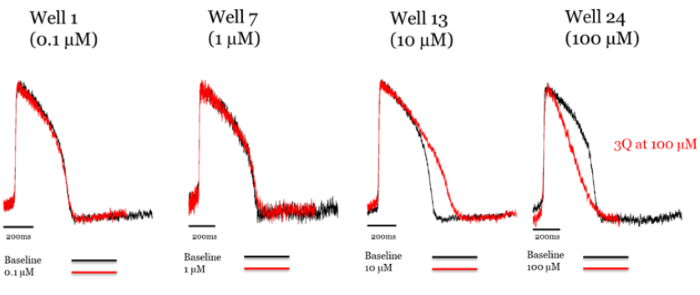
Figure 3. Addition of ranolazine to axoCells ventricular cardiomyocytes produces hERG blockade at 10 μM. Raw trace comparing control (black) to treatment with ranolazine (red). Image Credit: Axol Bioscience Ltd
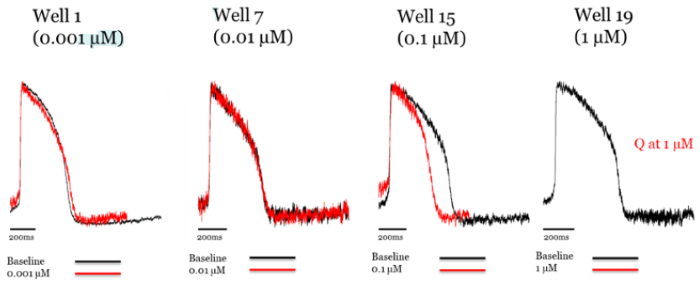
Figure 4. Addition of verapamil to axoCells ventricular cardiomyocytes produces hERG blockade at low concentrations, with L type Ca2+ channel block at higher concentrations. Raw trace comparing control (black) to treatment with verapamil (red). Image Credit: Axol Bioscience Ltd
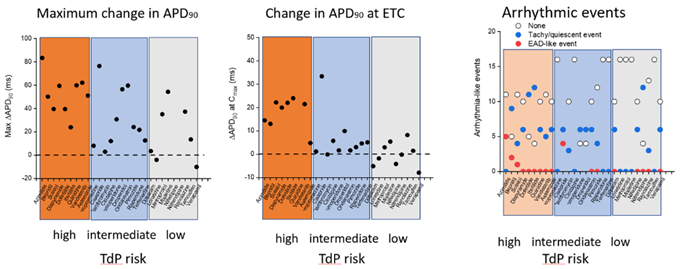
Figure 5. Summary data for all 28 CiPA compounds showing (left) maximal change in APD 90 , centre ) change in APD 90 at therapeutic concentrations , and (right) evidence of any arrhythmic events including EADs and quiescent events. Image Credit: Axol Bioscience Ltd
Discussion
An external partner was sought for an independent evaluation of axoCells ventricular cardiomyocytes for in vitro cardiac safety screening. The cardiomyocytes were tested against the 28 compounds with previously-defined torsadogenic risk that constitute the CiPA assay, with responses at rising concentrations assessed compared to the known compound effects.
axoCells ventricular cardiomyocytes yielded a predictable pharmacological response from all 28 CiPA compounds, thus validating their use in models of cardiotoxicity screening.
axoCells hiPSC-derived ventricular cardiomyocytes express numerous key ion channels and can respond to calcium channel blockers, including nifedipine and sodium channel blockers like terfenadine. Particularly, prolongation of the action potential duration, a proxy for QT prolongation, was visible in response to hERG block.
Of note, a graded response was observed that corresponded to the risk profile of pre-defined compounds. axoCells ventricular cardiomyocytes can thus be utilized to detect hERG block and associated TdP risk. To conclude, axoCells ventricular cardiomyocytes have use cases for in vitro cardiac safety testing and detecting proarrhythmic risk of novel compounds.
References and further reading
- Francis Grafton, Jaclyn Ho, Sara Ranjbarvaziri, Farshad Farshidfar, Anastasiia Budan, Stephanie Steltzer, Mahnaz Maddah, Kevin E Loewke, Kristina Green, Snahel Patel, Tim Hoey, Mohammad Ali Mandegar (2021) Deep learning detects cardiotoxicity in a high-content screen with induced pluripotent stem cell-derived cardiomyocytes eLife 10:e68714 doi: https://elifesciences.org/articles/68714
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: New estimates of R&D costs. J Health Econ. 2016 May;47:20-33. doi: https://www.sciencedirect.com/science/article/abs/pii/S0167629616000291?via%3Dihub.
- Blinova K, Dang Q, Millard D, Smith G, Pierson J, Guo L, Brock M, Lu HR, Kraushaar U, Zeng H, Shi H, Zhang X, Sawada K, Osada T, Kanda Y, Sekino Y, Pang L, Feaster TK, Kettenhofen R, Stockbridge N, Strauss DG, Gintant G. International Multisite Study of Human-Induced Pluripotent Stem CellDerived Cardiomyocytes for Drug Proarrhythmic Potential Assessment. Cell Rep. 2018 Sep 25;24(13):3582-3592. doi: https://doi.org/10.1016/j.celrep.2018.08.079. PMID: 30257217; PMCID: PMC6226030.
- Pognan, F., Beilmann, M., Boonen, H.C.M. et al. The evolving role of investigative toxicology in the pharmaceutical industry. Nat Rev Drug Discov 22, 317–335 (2023). https://doi.org/10.1038/s41573-022-00633-x
About AXOL Biosciences
The first choice for high-quality, functionally relevant iPSC-derived cells.
With over a decade of experience, we’ve developed the manufacturing capabilities to produce high-quality, functional iPSC-derived cells with excellent consistency.
Your research can benefit from our quality-focused approach, with our catalog of robust, highly relevant iPSC-derived neurons and cardiomyocytes developed at our ISO 9001:2015-accredited production facility.
Our leading neuronal cell types include: cortical excitatory neurons, striatal neurons, cortical inhibitory interneurons, microglia, astrocytes, sensory neurons and motor neurons. We also provide high-quality atrial cardiomyocytes and ventricular cardiomyocytes, as well as made-to-order myotubes.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.