Human-induced pluripotent stem cells (iPSCs) can be utilized to study complex health conditions in in vitro platforms relevant to humans. It is possible to reprogram human iPSC lines from patient blood cells and fibroblasts and differentiate them into endpoint cells relevant to the central and peripheral nervous systems, the cardiovascular system, and skeletal muscle alike.
These iPSC-derived cells can be gathered from patients with complex conditions such as Amyotrophic Lateral Sclerosis (ALS), Alzheimer’s Disease, and Parkinson’s Disease, and used in in vitro models for research and discovering new drugs.1, 2 Merging these cells with micro-physiological systems (MPS) can improve mimicking of the relationship among human cell types in vivo, delivering more comprehensive in vitro representations of human disease.
In the following article, Axol Bioscience demonstrates how multiple MPS platforms with human iPSC-derived neuronal cell types can be used to develop models of neurodegenerative conditions and pain. The compatibility of iPSC-derived neuronal cell types and MPS platforms is essential for the success of instituting complex biological interactions. Axol Bioscience outlines a range of important parameters for making a successful culture using the MPS platforms. Targeted and established biocompatibility is required for approaches that assess cell-to-cell interactions using multimodal endpoints, such as immunocytochemistry, cytokine release, neurite outgrowth, and multi-electrode array (MEA).
This article provides examples of complex human iPSC-based models. These examples include 2D and 3D neuromuscular junction (NMJ) models that make use of iPSC-derived skeletal muscle and motor neurons (to research specific disease-relevant targets in ALS) and simple monoculture systems that use iPSC-derived motor neurons or sensory neurons for axotomy, pain and itch models for discovering new drugs.
Microphysiological systems and 3D organoid models
Multiple systems are now available to build a given tissue model. This article focuses on using 3 distinct systems: standard microfluidic molded devices, 3D bio-printed scaffolds to operate with multi-electrode array instruments, and complex microfluidic systems with integrated MEA technology.

Figure 1. Overview of MPS platforms we currently use: 2D models using standard microfluidic commercial devices, 3D MEA using Axol Bioscience’s proprietary cell scaffold in combination with the Axion Maestro Pro MEA system, and 2D MEA, using the NETRI NeurofluidicsTM DuaLink device with the Axion Maestro Pro MEA system. Image Credit: Axol Bioscience Ltd
Key considerations for bio-compatibility
The biocompatibility of the MPS and certain cell types is a crucial point to consider. It is critical for customizing the iPSC-derived neuronal cell types and can improve the reliability and consistency of in vitro models.
Factors for consideration include:
Source: Axol Bioscience Ltd
| Impacting factors |
Affected outputs |
| Extracellular matrix or matrices used |
Cell adherence, function, cell longevity |
| Platform surface chemistry and adaptation |
Cell adherence, cell distribution, neurite outgrowth |
| Plating processes and cell density |
|
| Post thaw viability of cells |
|
Extracellular matrix evaluation
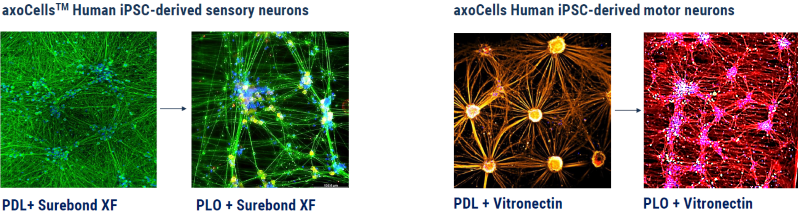
Figure 2. Comparison of different matrix coatings for both axoCells sensory neurons and motor neurons, displaying how different coatings can influence neuronal morphology and formation. PDL= Poly-D-Lysine, PLO= Poly-L-Ornithine. Image Credit: Axol Bioscience Ltd
Model surface chemistry
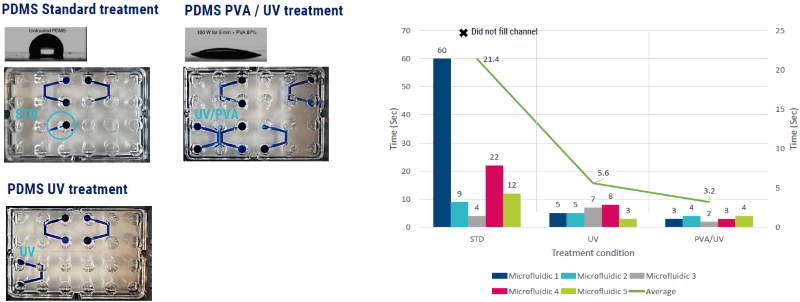
Figure 3. Specific treatments to the surface chemistry of an MPS system can change the flow dynamics and surface tension of a cellular solution inserted into the device. Treatment with UV and or PVA solution on PDMS microfluidic molds completely alters the surface tension and flow of a solution by converting a highly hydrophobic surface to hydrophilic. PDMS = polydimethylsiloxane. Image Credit: Axol Bioscience Ltd
Assessment of cell performance: MPS compatibility
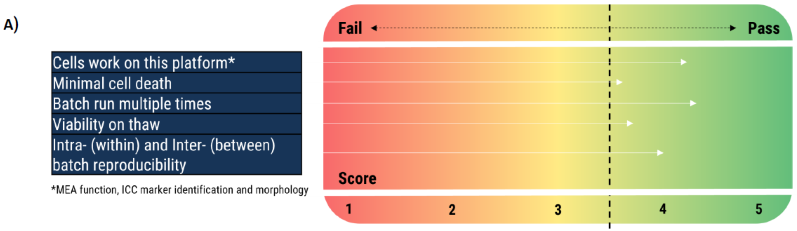
Image Credit: Axol Bioscience Ltd
Establishment of measurable criteria
| Cell type |
Matrices |
Morphology |
Marker expression |
Function |
Sensory neuron
Morphological and functional performance uniform across both ECM. |
PDL + SXF |
|
ẞ3 Tubulin ✓
Nav1.7 ✓
Nav1.8 ✓
VR1 ✓ |
MEA |
| Poly-Ornithine+SureBond-XF |
|
Motor neuron
Matrices induce both different morphological and functional characteristics in the motor neuron culture. |
Vitronectin + SureBond-XF |
|
ẞ3 Tubulin ✓
Hb9 ✓
CHAT ✓
ISL-1 ✓ |
MEA
<Synchronicity
<Network burst firing |
| Poly-Ornithine+SureBond -XF |
|
Figure 4. (A) Strategic approach to measuring cell performance across cell culture platforms or MPS devices. On this matrix, a score of 3.5+ is needed to pass each criterion. (B) A potential model for measurable criteria associated with the neuronal cells and the platform to validate a batch of cells against. Image Credit: Axol Bioscience Ltd
Standard MPS platforms with platform-specific, operationally validated neuronal systems
Properly validated and highly functional cell types can be utilized to develop sophisticated in vitro models with multifaceted end-point readouts. Building these as mono-, co-or even tri-culture with utility in multiple platforms is possible.
Axol Bioscience has utilized this method to build biological models in diverse MPS platforms with rapid maturation.
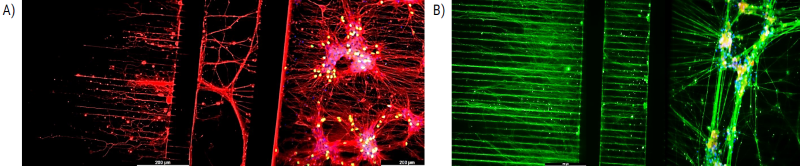
Figure 5. 2D model: NETRI Microfluidic devices built with axoCells motor neuron and sensory neuron cultures. (A) axoCells iPSC-derived motor neurons at day 10. Red = Beta tubulin marker, yellow = ChAT marker. (B) axoCells iPSC-derived sensory neurons at day 20. Green = Beta tubulin marker, yellow = Nav 1.7 marker, blue = Dapi. Image Credit: Axol Bioscience Ltd
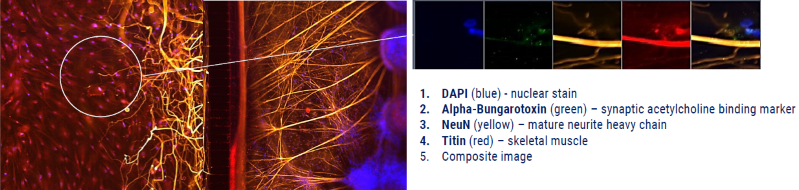
Figure 6. 2D model: Neuromuscular junction (NMJ) built with axoCells motor neurons and skeletal muscle in co-culture. Xona microfluidics co-culture of axoCells human iPSC-derived motor neurons and skeletal muscle as an NMJ model. The motor neurons (stained yellow using NeuN) completely overlap the postsynaptic acetylcholine receptors (stained green using fluorescent α bungarotoxin conjugates) and skeletal muscle (stained red using Titin). Image Credit: Axol Bioscience Ltd
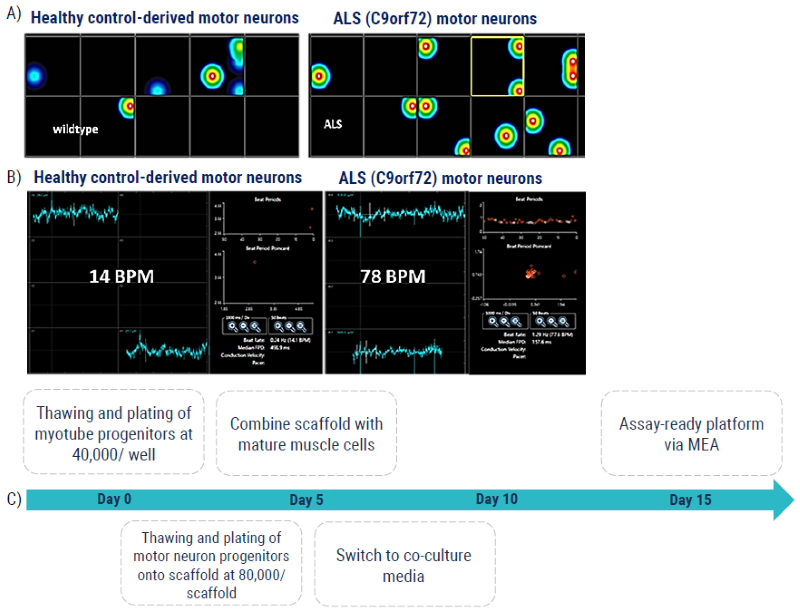
Figure 7. 3D Model: MEA NMJ model measuring motor neuron-induced skeletal muscle contraction for ALS. (A) The MEA platform measures skeletal muscle contraction driven by innervated motor neurons from the scaffold above, where neurites span a 50um fluidic space between the cell types. (B) The C9orf72 hyperexcitability phenotype of the ALS motor neurons is demonstrated here by the increased number of driven contractions (“beats”) per minute, BPM, compared to the healthy control-derived motor neurons. (C) Schematic timeline of the protocol used to generate the assay-ready platform in 15 days. Image Credit: Axol Bioscience Ltd
Conclusion
Developing helpful iPSC–based in vitro models for several platforms necessitates well-verified cell types that have been evaluated with multiple parameters for optimum functionality in the selected platform. This article has discussed key parameters to consider when validating human iPSC-derived neuronal cells and data on potential in vitro models that can be developed using these cells.
Building isolated 2D and 3D culture environments provides distinct approaches for assessing intracell interactions, such as an in vitro neuromuscular junction model. Fueling these culture environments with functional human iPSC-derived cell types has allowed researchers to generate more sophisticated in vitro models relevant to humans for research and discovering new drugs, paving the way for more effective therapies for patients worldwide.
Download the Poster Version
References and further reading
- Nicholson, M., Ting, C., Chan., et al. Utility of iPSC derived cells for disease modelling, drug development, and cell therapy. Cells 11, 1853 (2022). https://pubmed.ncbi.nlm.nih.gov/35681550/
- enney, J. et al. Modelling Alzheimer’s disease with iPSC-derived brain cells. Mol Psychiatry 25, 148–167 (2020). https://doi.org/10.1038/s41380-019-0468-3
About AXOL Bioscience
The first choice for high-quality, functionally relevant iPSC-derived cells.
With over a decade of experience, we’ve developed the manufacturing capabilities to produce high-quality, functional iPSC-derived cells with excellent consistency.
Your research can benefit from our quality-focused approach, with our catalog of robust, highly relevant iPSC-derived neurons and cardiomyocytes developed at our ISO 9001:2015-accredited production facility.
Our leading neuronal cell types include: cortical excitatory neurons, striatal neurons, cortical inhibitory interneurons, microglia, astrocytes, sensory neurons and motor neurons. We also provide high-quality atrial cardiomyocytes and ventricular cardiomyocytes, as well as made-to-order myotubes.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.