Sponsored Content by InProcess-LSPReviewed by Louis CastelNov 6 2024
M. Hermes, A. Grau-Carbonell, F. van Keulen , C. Schuurmans, A. Gerich, R. Besseling, InProcess-LSP, The Netherlands
Abstract
Particle size characterization in nanosuspensions often focuses on ‘majority’ features of the size distribution. Yet, for various suspensions in (bio)pharmaceuticals or fine chemicals, small fractions of ‘oversized’ particles can significantly impact their quality and safety. Efficient characterization of these particles -especially during suspension processing- remains a challenge. Here, a new mode is introduced to the established in/on/at-line size characterization capabilities of SR-DLS instruments (NanoFlowSizer): ‘Large Particle Detection’ (LPD). It provides rapid information of ‘rare’ aggregates and oversizers using high-speed cross-sectional video-imaging. The combined information from SR-DLS and LPD significantly extends current non-invasive measurement capabilities for nanosuspensions.
Introduction
Nanoparticle (NP) suspensions have become widespread in various industries, such as (bio)pharmaceuticals, specialty chemicals and cosmetics, either as final products or intermediates. In NP suspension development and manufacturing, real-time in/online monitoring of particle size characteristics via ‘Process Analytical Technology’ (PAT) is crucial for R&D efficiency and product quality in manufacturing. Longstanding limitations on such real-time, inline/non-invasive measurements have been overcome by using Spatially Resolved Dynamic Light Scattering (SR-DLS) based instruments (NanoFlowSizer, NFS) which measure NP size characteristics in flow and over vast turbidity ranges using >1000 simultaneous DLS measurements resolved over a few mm depth in the suspension. This has enabled PAT and QbD approaches to be applied and leveraged for R&D and production of nanomedicine 1,2,3 and other NP based products.
Often, NP size characterization focuses on the main features of the particle size distribution (PSD) such as the mean size, width and modality. However, ‘large’ particles constituting a very small fraction of the PSD (the ‘tail’) may be critical for product performance. Examples in nanomedicine include protein aggregates in biopharmaceuticals 4,5 or droplets larger than ~5 mm in lipid nano-emulsions (U.S.P. 729, 3), both of which compromise safety and efficacy; in high-performance inks or polishing slurries 6,7,8, particles > 0.5-1 mm are also highly undesired. Characterization of such ‘aggregates’ or ‘oversizers’ is a significant challenge, and typically needs off-line analytical tools, extensive sample handling and time-consuming method development, e.g. Single Particle Optical Sizing 9 Nanoparticle Tracking Analysis 10, coulter counting or resistive pulse sensing 11. Non-invasive or inline characterization possibilities, to enable real-time PAT or high throughput characterization of small aggregate or oversizer fractions, are thus highly desired.
Extending non-invasive SR-DLS with ‘Large Particle Detection’ (LPD)
The non-invasive SR-DLS technology of the NFS (Figure 1A) employs Low Coherence Interferometry, whereby backscattered light from a fixed beam (green line Figure 1C) is ‘instantaneously’ resolved over a few mm depth in the suspension with a few mm depth resolution. Depth-resolved flow correction 12 and/or spatial filtering of SR-DLS data are then used to circumvent biases in NP size measurement that can occur in standard DLS due to flow or high turbidity. Figures 1B and C show inline data during circulation of an aggregating Bovine Serum Albumin solution (5 mg/ml), with two main features: (i) the Z-average mean size (Figure 1B) systematically increases with time (ii) the cumulative PSDs (Figure 1C) show, in addition to the ~6 nm ‘monomer’ fraction in the bare PSD, a significant fraction of particles larger than ~ 1 mm. These large aggregates are mostly responsible for the increase in mean particle size, but they are difficult to characterize accurately in both SR-DLS under flow conditions and in standard DLS due to their low number.
![A) NFS probe equipped with a ½ inch flow cell as used inline. B) Z-Average particle size measured inline using the recently introduced PhaSR-DLS mode of the NFS [13], tracking heat-induced BSA protein aggregation during flow. C) The particle size distribution (PSD) and cumulative PSD measured in flow using PhaSR-DLS, after 30 minutes of heating. D) a single frame from an LPD movie captured during flow, around the same time point (30 min). For SR-DLS measurements the beam is fixed (green line), while for LPD measurements the beam is scanned to create the image (repeatedly for image sequences).](https://www.news-medical.net/images/Article_Images/ImageForArticle_25441_45591091688759041.jpg)
Figure 1. A) NFS probe equipped with a ½ inch flow cell as used inline. B) Z-Average particle size measured inline using the recently introduced PhaSR-DLS mode of the NFS 13, tracking heat-induced BSA protein aggregation during flow. C) The particle size distribution (PSD) and cumulative PSD measured in flow using PhaSR-DLS, after 30 minutes of heating. D) a single frame from an LPD movie captured during flow, around the same time point (30 min). For SR-DLS measurements the beam is fixed (green line), while for LPD measurements the beam is scanned to create the image (repeatedly for image sequences). Image Credit: InProcess-LSP
To enable non-invasive/inline characterization of small concentrations of ‘oversizers’, a ‘Large Particle Detection’ (LPD) imaging mode has been introduced in the NFS systems, see Figure 1D. In the LPD mode, high-speed lateral scanning of the beam yields ‘Optical Coherence Tomography’ cross-sectional images and videos (by repeated scanning), which may be alternated -on a ~30 second time scale- with (Pha)SR-DLS measurements. Typical images or videos span 5-10 mm in width, over a depth of ~1-3 mm depending on the NFS configuration. Images as in Figure 1D are processed to remove intrinsic reflections from the container walls (e.g. flow cell or vial) and are compensated for intensity gradients intrinsic to the optics and the LCI-detection scheme of the NFS 14.
While in SR-DLS measurements as in Figure 1B, the signal from each pixel is typically due to ‘coherent’ scattering from several small NPs in each pixel, scattering from a single particle can also be resolved when its size and optical contrast are sufficient. Such particles therefore appear as bright spots in the image: in Figure 1D these are clearly visible and indicate small quantities of large protein aggregates that are poorly resolved in SR-DLS or DLS. Since typical volumes of a single image are ~0.1-0.5 mL and image acquisition rates are ~100 Hz or beyond, LPD in principle allows full scanning of volumes up to ~50 mL/s. As shown below this has significant benefits.
Further characterization of the large particle contribution employs intensity histograms of processed LPD videos acquired over a user-specified timespan. These histograms form the basis for detecting very small fractions of particles that exceed a specified intensity threshold. This yields a characteristic similar to particle counts in SPOS or Coulter counting, but with the benefit of direct non-invasive inline application and ‘simultaneous’ SR-DLS data on the ‘majority’ PSD characteristics. SR-DLS supplemented by LPD thus provides a new level of PAT nanosuspension characterization.
Polystyrene particles
To illustrate detection capabilities of LPD, Figure 2 shows measurements of aqueous suspensions of 2 mm polystyrene particles with known concentrations. The data in Figure 2 A-D are snapshots of ~6 seconds movies on static samples in vials but results were identical in flow within the limits specified later. At concentrations << 104 particles/ml, individual images did not show signs of particles. However, for concentrations >104/ml, individual particles are clearly discerned in Figure 2 A-D with systematically increasing concentration. 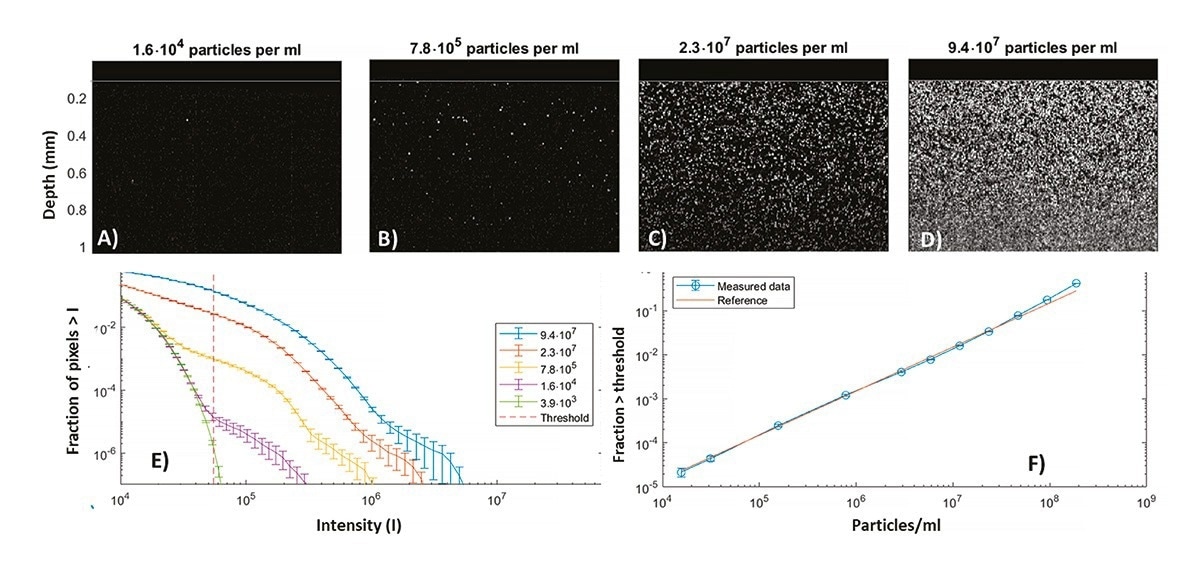
Figure 2. A)-D) Processed LPD images for 2 mm polystyrene particles at various concentrations. E) Corresponding histograms of fraction of pixels above a given intensity as function of intensity, obtained from ~1 min of imaging. Error bars show the standard error. f) Calibration curve showing the fraction of pixels brighter than the threshold of 5 x 104, as function of the reference concentration. Image Credit: InProcess-LSP
To analyse the data, intensity statistics were collected from repeated image sequences (10 videos, in total ~1 minute) for each concentration. From these statistics, histograms were generated, showing the fraction of pixels exceeding a given intensity as a function of intensity, shown in Figure 2D. At concentrations well below 104 particles/ml, the data reflect the instrument noise. However, at higher concentrations, the presence of particles becomes evident as a ‘shoulder’ in the data and the fraction of high-intensity pixels systematically increases with concentration.
For further analysis, a specific threshold above the noise level (here I* =5 · 104) is used and the corresponding fraction of pixels (fI*) is shown versus concentration c in Figure 2F. A linear dependence is observed (<5 % error) from c~104 to 3·107 particles/ml, and a threshold-specific calibration constant (c = 0.67 · 109 · fI*) allows to accurately infer the particle concentration. A notable feature is the remarkably low concentration of ~104 /ml (roughly 100x smaller than typical e.g. for NTA.) that is measurable for the present one minute measurement time. Analysis of even smaller concentrations is possible when the acquisition time (or flow rate in an inline situation) is extended. For the highest concentrations above 3·107/ml, some deviation is observed due to occasional appearance of more than one particle per pixel.
Application ranges of LPD
Using polystyrene particles of different size, and analyses as in the previous section, the concentration range for LPD using the NFS-Fides instrument has been evaluated in the size range 0.34-15 µm, see Figure 3A. Note that for each particle size a distinct threshold and calibration constant were employed. The blue-filled circles in Figure 3 indicate concentrations with an accuracy after calibration within 5 % of the reference value, for the present ~1 minute total measurement time. Open circles show low concentrations where measurement uncertainty exceeds ~20 %. For 800 nm particles this occurs at a roughly 10x larger concentration than for 2 µm particles, and for 340 nm particles at roughly 100x larger concentration. This is related to detection noise and may be overcome by employing longer duration measurements or different (flow) conditions, as shown by the shading in the figure. The maximum concentration that can be measured (⪆ 107/ml) increases slightly for smaller sizes, a result of differences in how temporal ‘double occupancy’ of pixels affects the intensity data.
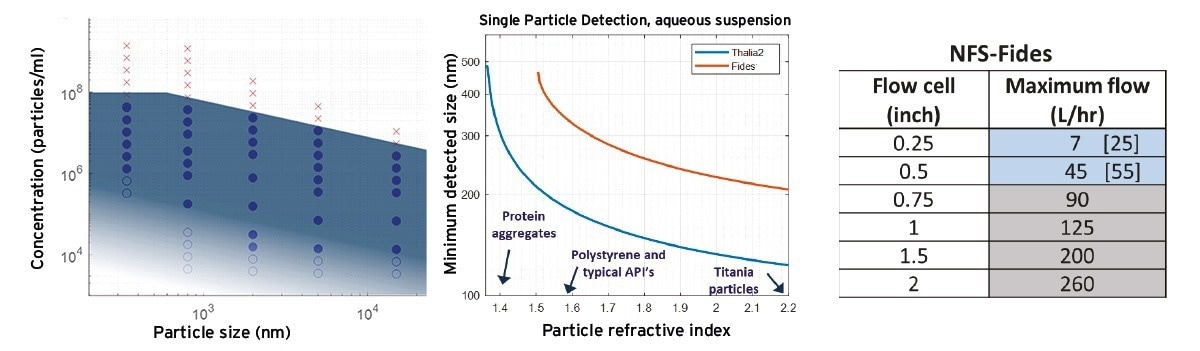
Figure 3. A) Indicative application range of LPD using the NFS-Fides system, for polystyrene, using data in Figure 2 for particle sizes from 0.34-15 µm. Filled circles: concentrations measurable at <5 % uncertainty, open circles: > 20 % uncertainty. Shading indicates that lower concentrations can be achieved using longer measurements. Red crosses: data exceeding the maximum concentration for LPD. B) The minimum detectable size versus particle RI (using water as dispersant), for the NFS-Fides system (red) used in fig.A) and for the high sensitivity NFS-Thalia2 system (blue). C) Indicative maximum flow rates for LPD for the NFS-Fides at medium sensitivity. Blue cells: limits linked to the imaging ‘line-rate’ (image ‘smearing’); values in brackets indicate limits when the maximum line-rate suffices (high contrast particles). Gray cells: flow rates corresponding to onset of turbulence. Image Credit: InProcess-LSP
Size detection limits Since (back)scattering for different types of particles depends both on their size and their refractive index (RI) relative to that of the dispersant, the minimum detectable size varies for different NPs, as in any ‘Single Particle’ method. This is shown in Figure 3B, where the red curve for the present NFS-Fides data shows the polystyrene limit of ~300 nm, increasing to over 500 nm for small contrast particles such as ‘non-compact’ protein aggregates with an effective RI of ~1.4. The blue curve in Figure 3B shows the results for the high-sensitivity NFS-Thalia2 (blue), where for RI > 1.55 the limit <200 nm. When a ‘background’ of smaller particles is present, detection limits increase, depending on the size, concentration and RI contrast. When such a background plays a significant role, for more concentrated samples, the ‘Online Micro Dilution’ unit 15 may also be employed in conjunction with NFS-LPD to resolve particles of interest.
Maximum flow rate: LPD measurements can be performed in flow as long as the flow is laminar and the line acquisition rate during imaging is sufficient to prevent ‘smearing’. For medium sensitivity settings of the NFS-Fides (~10 kHz line rate, as used for ≤ 800 nm data in Figure 3A), indicative maximum flow rates for different flow cells are shown in Figure 3C. For the smallest flow cells, image smearing due to high flow is the limiting factor (the limit may be raised for lower sensitivity requirements). For larger flow cells (grey), flow limits mark the onset of turbulence. Note that the current flow limits exceed the typical flow limits for SR-DLS 12, particularly for large particles. LPD can thus generally be applied inline alongside SR-DLS.
Monitoring protein aggregation
As discussed before, large protein aggregates present in small concentrations are hard to characterize accurately by DLS as well as SR-DLS, making inline aggregate information challenging to obtain. Thus, LPD protein aggregate monitoring can provide a valuable extension to the usual inline SR-DLS characterization of monomers ( Figure 1) or small aggregates at higher concentration. Here the inline monitoring is demonstrated for the process of heat-induced aggregation of BSA proteins as mentioned in Figure 1. Briefly, a solution of 5 mg/ml BSA in 10 mM NaCl was gently stirred in a flask, from which the solution was peristaltically circulated at 18 ml/min (inner tubing diameter 1.2 mm) through a 0.5 inch flow cell connected to the NFS-Fides for measurements in flow. Heating of the flask to 65 °C started after 30 minutes (t = 0), with 10 LPD measurements of approximately 6 seconds made every 5 minutes.
Figure 4 A-D shows snapshots of the BSA protein solution over time. In Figure 5A, taken at the onset of heating, only a few bright spots are visible. As heating progresses, Figures 5 B-D, corresponding to 30, 60, and 90 minutes respectively, a clear increase in the number of visible aggregates occurs. Additionally, there is a notable rise in the background brightness. The latter corresponds to an increase in scattering due to growth of small protein aggregates, below the LPD detection limit, but resolved via PhaSR-DLS, see 13. This background contribution is also visible in the histograms in Figure 4E, showing an increase in their fraction for intensities below ~ 104.
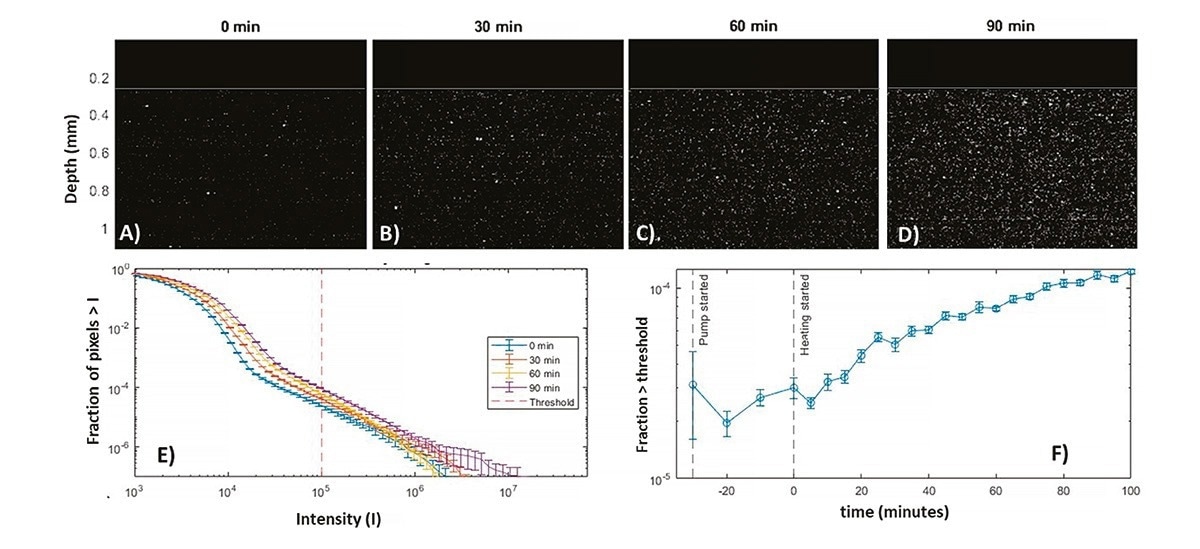
Figure 4. BSA heat induced aggregation measured during circulation from a flask at 18 ml/min using the NFS-Fides with a ½ inch flow cell. Pumping started at -30 minutes and heating at 0 minutes. A)-D) Scaled LPD snapshots with time lags after the start of heating shown on the top. E) Corresponding histograms of fraction of pixels above a given intensity as function of intensity, obtained from ~ one minute of imaging. F) LPD pixel fraction for a threshold 105 highlighting the growth of large aggregates. Image Credit: InProcess-LSP
The tails of the histograms in Figure 4E highlight the growth and increase in the fraction of large aggregates. To zoom in on these, a threshold of 105 was employed, and the corresponding pixel fraction is shown in Figure 4F. Already before heating, a slight increase in the large aggregate fraction is observed, likely due to the process of peristaltic pumping alone, whereby shear forces exerted in the tubing can cause aggregates to form. Upon initiating the heating process, the rate of aggregation and aggregate fraction increases significantly, quantitatively corroborating the visual observations in Figures 4A-D. There is no indication for saturation of this trend after 100 minutes of circulation, which is expected since a significant ‘monomer’ and small aggregate ‘source’ fraction is still observed in the PhaSR-DLS measurements.
While LPD currently does not inherently provide detailed size or size distribution data for the aggregates (without additional calibration), it can effectively capture and compare sample trends over time. For the present data and thresholds used in Figure 4F, it is estimated that detected aggregates exceed ~ 4 mm in size. The LPD characteristics can therefore be employed as characteristic fingerprint of the process, particularly useful during R&D and process development. For particles with well-known refractive index, the use of calibration would allow to infer more quantitative size information.
Conclusion
A novel extension of inline, non-invasive nanoparticle size characterization using the NanoFlowSizer (NFS) has been introduced: ‘Large Particle Detection’ (LPD) via high-speed cross-sectional imaging. Using this measurement mode, ‘rare’ large particles in the size distribution, occurring at fractions previously inaccessible for inline SR-DLS monitoring (or in standard off-line DLS) can be characterized. Using polystyrene standards, it is demonstrated that fractions down to roughly 104 particles/ml can be characterized efficiently (within one minute), while qualitative monitoring of the heat-induced growth of large protein aggregates has been illustrated during inline measurement. The innovative combination of SR-DLS nanoparticle sizing with the new LPD capability thus opens up new PAT monitoring possibilities for nanosuspension characterization. Besides aggregate detection, LPD also allows diagnostic of bubbles or undesired ‘large scale’ suspension inhomogeneities and thereby provides a useful inline trouble shooting tool during R&D and process development.
References
- Sheybanifard, M., et al. (2022). Liposome manufacturing under continuous flow conditions: towards a fully integrated set-up with in-line control of critical quality attributes. Lab on a Chip, [online] 23(1), pp.182–194. https://doi.org/10.1039/D2LC00463A.
- Matthessen, R., et al. (2024). Impact of mixing and shaking on mRNA-LNP drug product quality characteristics. Scientific Reports, [online] 14(1). https://doi.org/10.1038/s41598-024-70680-4.
- Rooimans, T., et al. (2023). Development of a compounded propofol nanoemulsion using multiple non-invasive process analytical technologies. International Journal of Pharmaceutics, 640, p.122960. https://doi.org/10.1016/j.ijpharm.2023.122960.
- Scherer, T.M., et al. (2012). Issues and Challenges of Subvisible and Submicron Particulate Analysis in Protein Solutions. The AAPS Journal, 14(2), pp.236–243. https://doi.org/10.1208/s12248-012-9335-8.
- Ripple, D.C. (2011). Standards for the Optical Detection of Protein Particles. [online] Americanpharmaceuticalreview.com. Available at: https://www.americanpharmaceuticalreview.com/Featured-Articles/36988-Standards-for-the-Optical-Detection-of-Protein-Particles/ [Accessed 5 Nov. 2024].
- Kuntzsch, T., et al. (2003). Characterization of Slurries Used for Chemical-Mechanical Polishing (CMP) in the Semiconductor Industry. Chemical Engineering & Technology, 26(12), pp.1235–1239. https://doi.org/10.1002/ceat.200303050.
- G. Bahar Basim and Moudgil, B.M. (2002). Effect of Soft Agglomerates on CMP Slurry Performance. 256(1), pp.137–142. https://doi.org/10.1006/jcis.2002.8352.
- Seo, J. and Paik, U. (2016). Preparation and characterization of slurry for chemical mechanical planarization (CMP). pp.273–298. https://doi.org/10.1016/b978-0-08-100165-3.00011-5.
- Tolla, B. and Boldridge, D. (2010). Distortion of Single-Particle Optical Sensing (SPOS) Particle Count by Sub-Countable Particles. Particle & Particle Systems Characterization, 27(1-2), pp.21–31. https://doi.org/10.1002/ppsc.200900081.
- Filipe, V., Hawe, A. and Jiskoot, W. (2010). Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharmaceutical Research, [online] 27(5), pp.796–810. https://doi.org/10.1007/s11095-010-0073-2.
- Grabarek, A.D., et al. (2019). Critical Evaluation of Microfluidic Resistive Pulse Sensing for Quantification and Sizing of Nanometer- and Micrometer-Sized Particles in Biopharmaceutical Products. 108(1), pp.563–573. https://doi.org/10.1016/j.xphs.2018.08.020.
- Schuurmans, C. (2024) Inline particle sizing in flow for demanding nanosuspension processes. Available at: https://www.researchgate.net/publication/359691923 (Accessed: Sep. 24, 2024).
- Koumakis, N., et al. (2024) PhaSR-DLS’: a new advancement in Spatially Resolved DLS for enhanced inline and off-line nanoparticle sizing. Available at: https://www.news-medical.net/whitepaper/20241106/PhaSR-DLSe28099-A-new-advancement-in-spatially-resolved-DLS-for-enhanced-inline-and-off-line-nanoparticle-sizing.aspx (Accessed: Sep. 24, 2024).
- Ghafaryasl, B., (2018) Accurate estimation of the attenuation coefficient from axial point spread function corrected OCT scans of a single layer phantom. Available at: https://www.researchgate.net/publication/323190259_Accurate_estimation_of_the_attenuation_coefficient_from_axial_point_spread_function_corrected_OCT_scans_of_a_single_layer_phantom.
- AZoNano (2023). AZoNano. Available at: https://www.azonano.com/article.aspx?ArticleID=6442 (Accessed 5 Nov. 2024).
About InProcess-LSP
InProcess-LSP, headquartered in Oss at Pivot Park, is a rapidly growing, innovative company founded in 2014. Backed by a team of in-house experts—comprising physicists, chemists, and software engineers—InProcess-LSP is at the forefront of nanotechnology solutions. The company’s leading product, the NanoFlowSizer, is a cutting-edge instrument designed to deliver inline, real-time measurements of nanoparticles in solution, making it indispensable across various industries.
Utilizing Spatially Resolved Dynamic Light Scattering (SR-DLS) technology, the NanoFlowSizer enables accurate characterization of nanoparticles in flowing liquids, providing critical data such as hydrodynamic diameter, polydispersity index (PDI), and D90 within seconds.
This state-of-the-art instrument empowers both scientists and industries by offering a robust solution for analyzing nanoparticle properties, paving the way for breakthroughs in product development, improved formulations, and pioneering applications.
Innovators in process analytical technology and nanoparticle characterization.
With their strong background in process analytics as well as many years of academic and industrial experience InProcess offer a highly skilled and experienced team of scientists and process specialists addressing the needs of your PAT and nanotechnology challenges.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.