Sponsored Content by InProcess-LSPReviewed by Louis CastelNov 6 2024
Authors: Albert Grau-Carbonell, Yan Wang, Marko Verbeek, Remy van Tuijn, Carl Schuurmans, Rut Besseling, Ad Gerich
Abstract
Aim: To demonstrate the potential of in-line nanoparticle size measurements using the NanoFlowSizer (NFS) as a PAT method. To achieve real-time process control by establishing automated regulation of critical parameters during manufacturing.
Methods: A NanoFlowSizer was integrated into a High Pressure Homogenization (HPH) nanoemulsification process and a Turbulent jet Injection skid for continuous liposome manufacturing. Proportional-Integral-Derivative control was established by means of a customized controller module of the dedicated XsperGo software.
Conclusion: In-line particle size measurements by the NanoFlowSizer were successfully employed for PID control of critical process parameters (valve pressure, flow rates) to achieve real-time process control. An HPH process was automated by allowing the NanoFlowSizer to control electronically, via a PID controller, the pressure at the high-pressure valve of the system, and was automatically stopped at the desired particle size to avoid emulsion overprocessing. Also, a continuous liposome manufacturing process (via turbulent jet injection) was successfully controlled by allowing the NanoFlowSizer to take PID control of the aqueous flowrate.
Introduction
The final objective when integrating Process Analytical Technologies (PAT) is to acquire process control and, optionally, real-time release by automatically regulating Critical Process Parameters (CPPs). These CPPs are defined as the parameters that determine the final Critical Quality Attributes (CQA) of the product, those properties of that are critical for its performance for end-users or as intermediate products. Examples of CPPs are temperature or pressure during manufacturing, while examples of CQAs are particle size and composition.
In many liquid products containing nanoparticles, particle size is a CQA. Examples range from pharmaceutical suspensions (containing e.g. Lipid Nanoparticles [LNP], Liposomes, APIs) to suspensions used for energy technologies (precious metal or oxide nanoparticles). However, nanoparticle size has been a difficult parameter to monitor during processing. Furthermore, many processing techniques used to generate these products involve size reduction steps that are heavily influenced by the process conditions. Despite the significance of particle size on relevant properties of dispersions such as rheology, stability and safety, (Goodarzi and Zendehboudi 2019) a method to continuously measure particle size (inline or online) and potentially control the production process has been lacking, primarily due to previous limitations of sub-micron particle size measurements in flow and at high turbidity.
Recently, Spatially Resolved Dynamic Light Scattering (SR-DLS), the technology behind the NanoFlowSizer, has successfully tackled these issues for suspensions of sub-micron particles. (Besseling, Damen, et al. 2019) SR-DLS is a light scattering technique based on Fourier Domain Low-Coherence Interferometry (FDLCI), (Kalkman, Sprik and Leeuwen 2010). It resolves the scattered signal of an illuminated volume as function of depth in the sample. FDLC is routinely used in Optical Coherence Tomography (OCT) devices for medical imaging.
The NanoFlowSizer allows for the continuous measurement of particle size in the submicron regime at timescales compatible with manufacturing control: fast measurements in flow and at high turbidity. (Schuurmans, et al. 2022) Here, we present the integration of the NanoFlowSizer as a PAT tool for continuous particle size monitoring and automated control of CPP (pressure, injection flow rates) via feedback loops to achieve target particle sizes.
The NanoFlowSizer (SR-DLS)
The NanoFlowSizer is an analytical instrument to measure submicron particle size (Figure 1A). The measurements are based on novel Spatially Resolved Dynamic Light Scattering (SR-DLS).
Standard DLS is based on measuring the diffusion rate of particles suspended in a liquid by illuminating them and analyzing the fluctuations in scattered light signals. Suspended particles diffuse via Brownian motion, constantly changing their relative positions in the scattering volume. As a result, the scattered signal fluctuates at a rate which is proportional to the particle’s diffusion rate. By computing decorrelation functions of the scattered signal, the diffusion constant (or its distribution) can be measured, which can be translated into the hydrodynamic size of the diffusing particles via the Stokes-Einstein relation. (Einstein 1905).
When a fluid flows through a pipe, a flow profile is established (Figure 1B). If the flow is laminar, a well-defined parabolic velocity profile forms, with zero flow at the wall and a maximum velocity in the center of the pipe. Since standard DLS measurement and analysis requires a homogeneous suspension in which particle motion is limited to Brownian diffusion, the flow conditions sketched above make standard DLS measurements of flowing samples typically impossible.
SR-DLS circumvents these flow limitations by implementing Low Coherence Interferometry (LCI) methods (Figure 1C). In LCI, broadband light illuminates the samples and (180⁰) backscattered light is then mixed with the reference beam in an interferometer (Figure 1B). From the measured spectrum of this mixed signal (over all wavelengths of the light source), the scattered signals from different depths in the sample can be resolved simultaneously via a mathematical (Fourier) transform of the spectra. Typically, 1000 consecutive depths with a resolution of a few microns and total depth in the sample of a few millimetres are thus analyzed. Using ~50 kHz measurement rate, fluctuations of the scattered light at each depth are thus rapidly acquired and a decorrelation function can be computed for each depth (Figure 1D). These are then used to characterize the flow profile and apply flow corrections to the measured diffusivities at each sample depth, resulting in an accurate measurement of the particle’s Brownian diffusion constant (and therefore its hydrodynamic radius).
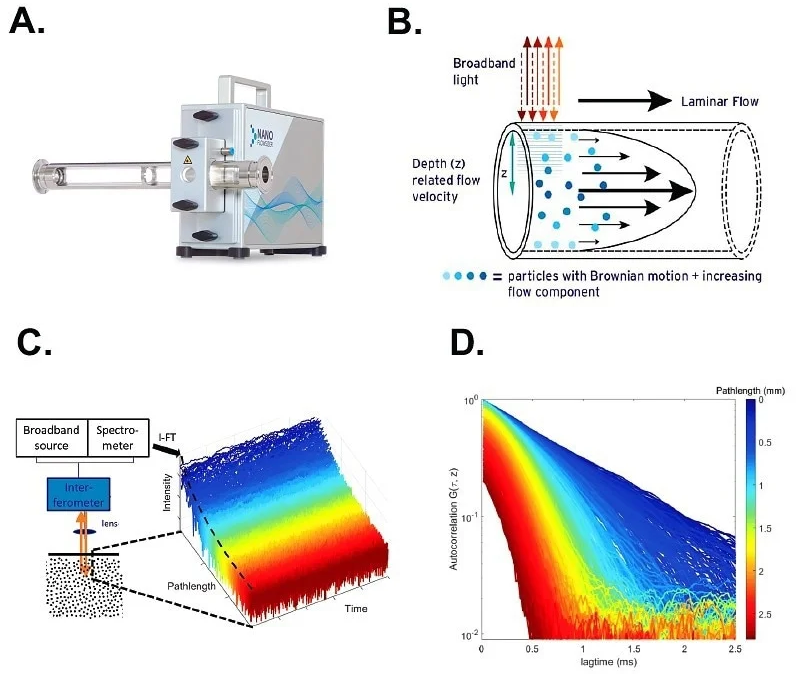
Figure 1. The NanoFlowSizer (NFS) and Spatially Resolved Dynamic Light Scattering (SR-DLS). A. Probe unit of the NFS, with a flow cell adaptor mounted to connect to a process for in-line or on-line particle sizing in flow. B. SR-DLS detects broadband light backscattered from a suspension via interferometry and resolves scattered light fluctuations, and thereby particle diffusion and flow, at different depths. For laminar flow, the velocity vanishes at the walls and is at its maximum in the middle of the tubing. C. Detail of the detection scheme: interferograms from the spectrometer are transformed to give the scattered light from each depth in the sample. High-speed acquisition thus yields the fluctuations of this scattered light at the different depths (blue: close to the wall, red: deeper in the suspension) D. Decorrelation functions at different depths (obtained simultaneously from fluctuations as in C) of a suspension flowing in a tube. Decay is faster at larger depths in the sample (red) indicating the higher flow velocity away from the wall. The flow profile can thus be analyzed and employed for flow correction, allowing correct analysis of the diffusion and particle size characteristics via the Einstein-Stokes equation. Image Credit: InProcess-LSP
Process automation via NanoFlowSizer
The NanoFlowSizer is used as a PAT tool to automate processes in which particle size is a CQA determined by a CPP. By measuring particle size in real time downstream after the critical process step that determines their size, feedback control can be established on that very process. The integration of an NFS in a process is only contingent on a few simple practical things: change in the particle size (distribution) should happen at size ranges, timescales and flowrates compatible with SR-DLS measurements, and the process step that determines the particle size change (e.g. high-pressure homogenization) should allow electronic, gradual control of the associated CPP.
A Proportional-Integral-Derivative controller (PID controller) is a convenient, commonly used method to integrate control loops into processes requiring automatic feedback control (Figure 2A). Proportional-only control is a PID iteration by which the difference between the target and currently monitored CQA determines a proportional change of the CPP under PID control, while Integral and Derivative adjustments are not used. This proportionality is manually determined with a proportional gain constant (Kp). Proportional-only is an excellent method for slow processes without overshooting or oscillating behaviors. Other control parameters that can be imposed are a maximum to the change that the CPP can undergo in a single change step, or an early stop margin value that stops CPP control when the CQA is sufficiently close to target, to avoid overprocessing (i.e. CQA overshooting). Typical challenges of basic PID control algorithms are noisy or imprecise measurement data, slow measurements that leads to lagged control, and highly non-linear processes that limit convergence on a CQA and may destabilize the system. However, as shown below, PID control of nanosuspension processes using the NFS did not suffer from these issues.
The NanoFlowSizer’s dedicated software XsperGo offers the possibility to connect to an external system and control its CPP via PLC communication (Figure 2B). Once this connection is established, XsperGo includes a customizable PID controller that can be readily configured. This module offers the ability to tune all typical PID parameters for automated control of linear processes by measuring average particle size. Other characteristics of the size distribution from SR-DLS measurements, such as the Polydispersity Index (PdI) or the size that covers 90% of the volume of particles (DV90), can also be used as CQA for PID control if their measurement precision for a typical process is good enough.
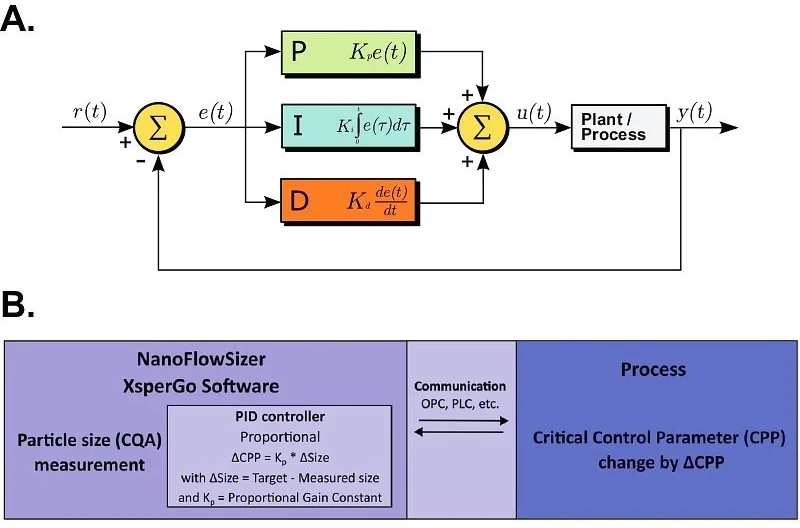
Figure 2. Proportional-Integral-Derivative (PID) process control and NanoFlowSizer implementation. A. The operation of a PID controller is based on changes of a critical control parameter (CPP) of a process by measuring Critical Quality Attributes (CQA) and comparing the measurements to a target value. ‘Proportional control’ relies on CPP changes (‘u’) proportional (by a factor Kp) to the difference ‘e’ between measured (y) and target value (r). Integral control relies on integrating the difference to set the CPP change, while Derivative control employs the rate of change of the difference (Wikipedia 2023). B. PID control can be readily implemented using the NanoFlowSizer. A PID control module, part of the XsperGo software, allows to establish communication between process equipment and the NFS for direct CPP control based on particle size measurements. A conceptual example of a simple Proportional control is shown, in which Kp is defined in XsperGo and communication is established via PLC with a process. Image Credit: InProcess-LSP
The NanoFlowSizer as a PAT tool for High-Pressure Homogenization (HPH)
High-Pressure Homogenization (HPH)
High-Pressure Homogenization is a well-established method for size reduction and homogenization of droplets in crude stock emulsions to a final homogeneous emulsion of nano-sized droplets. During HPH, an emulsion passes through the homogenization system, either once or repeatedly until a target droplet size is achieved. During each pass, the dispersion is exposed to very high shear conditions due to local high pressure (several kbar) in the system, resulting in a further homogenized sample (monodisperse, convergently reduced particle size). Importantly, this high pressure is the main parameter in determining particle size after a pass through an HPH, and thus an ideal candidate for automated control of the homogenization process. Understanding the impact of pressure changes for each specific formulation is a complex topic (Gupta, et al. 2016) and much work is required to understand it fully. (Håkansson 2019). Furthermore, a common issue with HPH (and most size-reduction methods) is overprocessing, in which a suspension is passed an excessive number of times through the size-reduction process, which can cause coalescence and instabilities in the product. Along with the associated energy waste and costs (HPH consumes significant energy), overprocessing thus has multiple undesired effects. Periodically monitoring the droplet size may prevent overprocessing. Still, the current manual sampling and measurement methods severely limit the frequency and convenience: manual sampling requires development of sampling methods and dilution steps, which may cause artifacts and time constraints. Instead, inline or online real-time measurements using the NFS as PAT tool are much more optimal. Moreover, combined with the NFS’ PID control and HPH interfacing, the continuous monitoring allows automatic tuning of the HPH pressure and achieves fast approach to the target droplet size.
Real-time control of HPH of emulsions via in-line continuous size characterization
Precise real-time control of an HPH nano emulsification process for a 5% oil-in-water emulsion was achieved by connecting a NanoFlowSizer directly to an HPH (GEA XStream Lab Homogenizer 1000) and integrating it in-line for droplet size measurements in a recirculation set-up (Figure 3A). The PID controller in XsperGo software was used to adjust the pressure at the high-pressure valve of the HPH via PLC communication. At the same time the sample was pumped from an external recirculation tank into the in-line NFS and back to the HPH (Figure 3B). A similar setup was previously used by us to continuously monitor particle size during HPH with manual pressure control. As opposed to manual pressure control, during which a given pressure was sustained until a particle size was reached and then shifted to a higher value in relatively large steps (Besseling, Arribas-Bueno, et al. 2023), PID control allowed for the gradual, continuous change of pressure for more rapid approach to the target size.
Increasing pressure in the high-pressure valve increased the local shear, reducing the resulting droplet size. XsperGo’s PID controller was customized to allow proportional pressure control, with a manually set proportionality constant (Kp = -0.13) and target particle size (200 nm). The NFS thus automatically tuned the pressure with a limit on the pressure change per step (20 bar). Using the difference between the continuously in-line measured droplet size and the target, the NFS gradually adjusted the pressure in the HPH to reach the desired target droplet size and automatically stopped the homogenization process (Figure 3C). This configuration proved effective, and any effects of the residence time of the sample in the HPH hopper, recirculation tank or in-line NanoFlowSizer set-up were not substantial.
Besides the mean (Z-average) droplet size, all other droplet size characteristics such as Polydispersity Index (PdI) and D90 were recorded as part of normal NFS operation (Figure 3D). D90 first dropped, as expected for initial coarse emulsion homogenization, and then decayed at a slower rate, as expected for the stochastic breakage of the remaining big emulsion droplets. The nearly flat PdI behavior, on the other hand, is due to changes in the size and width of the size distribution in combination with a complex relation between droplet size and (Mie) light scattering from the polydisperse distribution: a stable PdI during size reduction may still indicate (for some sizes and distributions) a narrowing of the particle size distribution. (InProcess-LSP 2023, ResearchGate 2023)
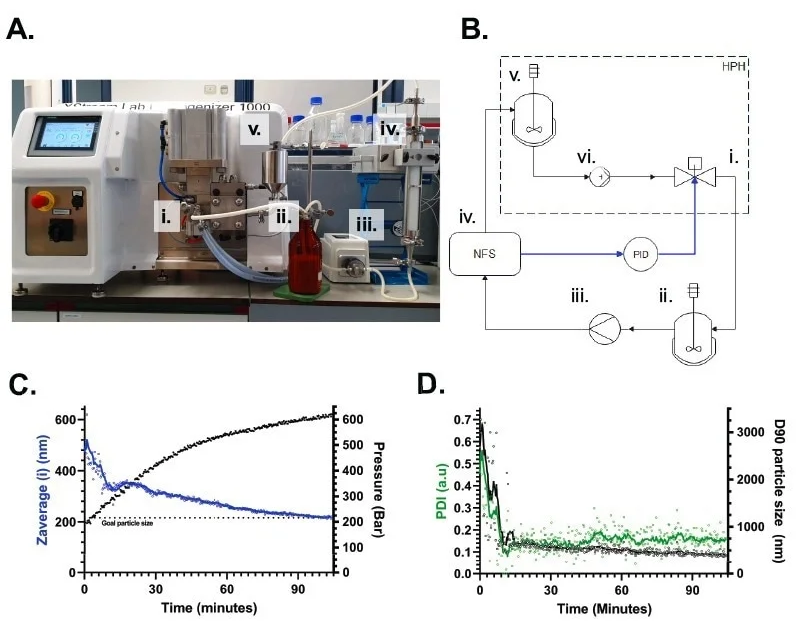
Figure 3. High-Pressure Homogenization (HPH) automated control via particle size measurement. A. Setup of a NanoFlowSizer for real-time droplet sizing, integrated in-line in a homogenization loop with a GEA Xstream Lab Homogenizer 1000 HPH with pressure controlled by the NFS-PID. B. Schematic of the HPH-NFS set-up. The PID feedback (blue arrows) allows NFS-control (iv) of the High-Pressure valve (i, HP-valve) to process the emulsion. Product is then directed to a recirculation tank (ii.) from which a micro gear pump(iii.) feeds the emulsion through the NanoFlowSizer flow cell (iv.) for measurement. After that, the emulsion returns to the HPH hopper (v.) from which a piston pump (vi.) directs it again to the HP-Valve. C. Particle size monitoring (10s/measurement and 10point moving average) and HPH pressure control (black) by the NFS during processing of an oil-in-water (5%) emulsion. The dotted line marks the Target Size input to the XsperGo PID controller (200 nm). D. Polydispersity Index (PdI) and D90 (intensity-based) of the emulsion corresponding to C). Both characteristics rapidly decreased in the first minutes as most droplets >2 µm were broken up in this timeframe. Lines: 10-point moving average. Image Credit: InProcess-LSP
The NanoFlowSizer as a PAT tool for turbulent jet Injection liposome manufacturing
Turbulent jet Injection for liposome production
Turbulent jet Injection is an established method to produce lipid-based nanoparticle, by injecting organic media (e.g. ethanol) with dissolved lipids into a flowing aqueous phase, which results in formation of the lipids into particles. (Costa, et al. 2015) The parameters that determine the size and polydispersity of the resulting liposomes for a given formulation are the Reynolds number of the aqueous-ethanol mixture flowing in the receiving pipe, the final particle chemical composition and the Flow Velocity Ratio (FVR) of the aqueous to the ethanol flow. (Costa, et al. 2015) This continuous method of producing liposomal particles presents many practical advantages over batch production (reduced energy usage and product waste combined with higher product quality and control). Given its continuous nature, PAT control is of particular interest, as particle size measurements can inform how to tune FVR to achieve a target particle size. The NanoFlowSizer is currently widely used for size measurements of lipid-based nanoparticles and can be used for integration in continuous liposome production as a PAT tool for automated process control.
Real-time control of turbulent Injection for continuous liposome manufacturing via in-line continuous size characterization
The NanoFlowSizer was successfully integrated into a LiFT liposome continuous manufacturing skid (DIANT Pharma Inc.) in an in-line configuration (Figure 4A). This system produces liposomes in a continuous mode by turbulent jet mixing of an aqueous and an ethanolic phase containing lipids. A PID controller in the DIANT Pharma Inc. LiFT skid, updated by the XsperGo software, was used to adjust the FVR by controlling the pump driving the aqueous flowrate of the turbulent jet injection step via OPC communication (Figure 4B). As a result, direct control of liposome size and polydispersity index was achieved. Importantly, for continuous manufacturing, the goal of PID control is not only to achieve a desired target size, but to adjust the system parameters continuously to ensure that the product CQA of choice, particle size in this case, is constantly at the desired value and within the required window. Using the NanoFlowSizer as a PAT tool to monitor and understand the liposome formation process falls directly in the realm of Quality by Design (QbD), as defined by the U.S. Food and Drug Administration (FDA).
PID control of the aqueous flowrate resulted in the rapid convergence of particle size to the target size of 85 nm (<10 minutes, Figure 4C). Liposome size was then maintained at the desired target by continuous PID control. This led to improved size stability in time as compared to continuous manufacturing without PID control. A supervised system led to a Z-average standard deviation of 3 nm, as compared to 4 nm for an unsupervised system, with a standard error of the mean of 0.1 nm for the supervised system compared to 0.2 nm for an unsupervised system. Using the NanoFlowSizer as PAT tool for PID control of the DIANT Pharma LiFT system not only allowed for the automation of flowrates to achieve a desired liposome size, but thus also improved the quality and consistency of the product by continuously fine-tuning the process.
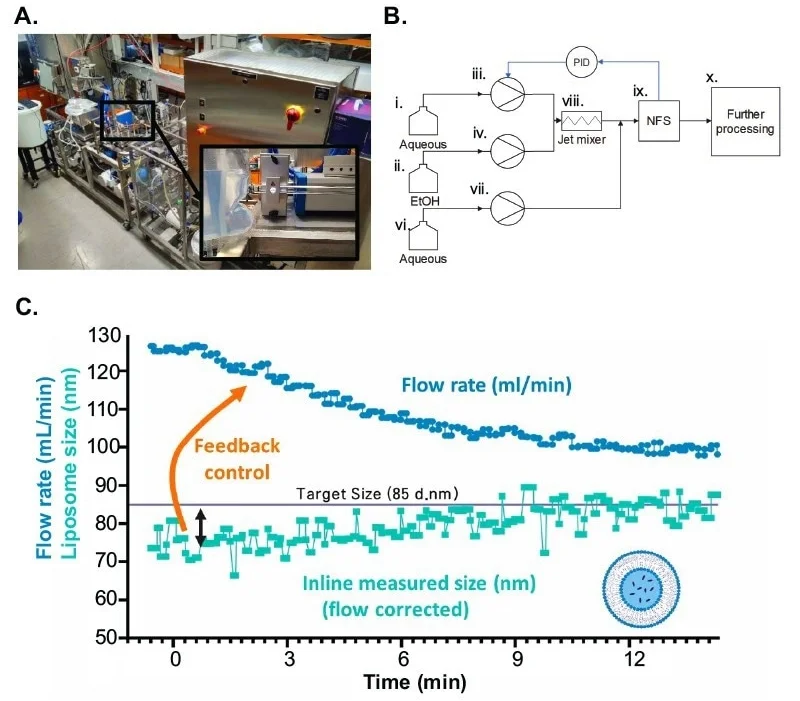
Figure 4. Turbulent Injection automated control via liposome size measurement. A. Set-up of an NFS integrated in liposome continuous manufacturing LiFT system (DIANT Pharma Inc.) in an in-line loop for real-time particle size measurement and automated feedback control of the internal aqueous flow rate. B. Schematic of the LiFT-NFS set-up. The aqueous (i) and the ethanolic (ii) phases were pumped (iii and iv) to the turbulent jet mixer (viii). Before entering the NanoFlowSIzer (ix), the suspension was diluted - required from product perspective, not for NFS measurement- from a secondary aqueous deposit (vi and vii). The PID feedback (blue arrows) allows NFS-control (ix) of the aqueous flow rate during turbulent jet injection (viii). The flow then continued for further processing (x) and final collection. C. Continuous, real-time measurement of liposome particle size (Z-Average) during automated aqueous flowrate regulation via PID control by a NanoFlowSizer. The line at 85 nm indicates the input target size in the PID controller of the LiFT system, updated continuously with the measured Z-average data from the NFS. The target size was achieved after 10 minutes after the start of the process and maintained at precisely 85 nm during continuous manufacturing. Aqueous flow rate as regulated by the NanoFlowSizer from the real-time particle size measurements. Flowrate was automatically tuned (from ~130 mL/min to ~100 mL/min) until the target liposome size was achieved, and it was continuously adapted to maintain the continuous liposome production at the target size. Image Credit: InProcess-LSP
Conclusion
Continuous process control via real-time particle size measurement was achieved for High-Pressure Homogenization (HPH) of emulsions and Turbulent Injection continuous manufacturing of liposomes. The NanoFlowSizer was integrated into the processes in an in-line configuration, and PID control was achieved via the dedicated PID module of the XsperGo software. Communication between the NanoFlowSizer and other equipment was established via PLC with a Profinet protocol.
For HPH (GEA XStream Lab Homogenizer 1000), the NanoFlowSizer allowed for the automated control of the internal pressure at the High-Pressure Valve of the system, thus controlling the resulting droplet size of a 5% oil-in-water emulsion. Furthermore, the process was automatically stopped at the desired size to avoid overprocessing. For the Turbulent jet Injection method for continuous liposome manufacturing, the NanoFlowSizer allowed for the automated adjustment of the aqueous flow rate in a LiFT skid system (DIANT Pharma Inc.), resulting in the rapid convergence and stability of liposome size to a target size value. This value was then sustained in time, leading to improved stability and product uniformity during continuous liposome manufacturing.
In conclusion, the NanoFlowSizer can be easily integrated into processes in which particle size is a CQA and can be used to tune CPPs such as pressure or flow rate. The dedicated XsperGo software allows for the easy deployment of PID control with customizable features to adapt to a multitude of needs, such as achieving a target size, avoidance of overprocessing or establishment of precise continuous manufacturing for real-time quality control.
Materials and methods
Materials
Sunflower oil (Sigma-Aldrich), Tween 20 (Sigma-Aldrich), water.
Standard NanoFlowSizer set-up for HPH
A FIDES I type NanoFlowSizer equipped with a 1.5-inch flow cell was connected in an in-line configuration (see Figure 3B). The connection between the system of interest and the flow cells was done via standard tri-clamps. The HPH was connected to a Recirculation Tank, from which the sample was pumped through the NanoFlowSizer by a micro gear pump (LongerPump, WT3000-1JA) and back to the hopper of the HPH. Flow rates for the micro gear pump were manually changed to match the process speed over time.
Emulsion preparation for HPH
A 5% mixture of sunflower oil in water was prepared with 1% Tween 20 as surfactant. The emulsion was pre-homogenized to 600 nm (at 200 bar) prior to the NanoFlowSizer homogenization run to set a standard known starting point.
NanoFlowSizer integration and control of HPH
An Ethernet LAN cable was used to connect the HPH PLC to the NanoFlowSizer PC, and a Profinet (TCP/IPv4) connection was established. The controller module of XsperGo software for HPH applications was then initiated. Proportional-only control was established with proportionality constants P (Kp): -0.13, I: 0 and D: 0, with a particle target size of 200 nm. Pressure changes were applied by the NanoFlowSizer every 2 minutes after size measurements. The initial pressure was set to 200 bar and the maximum possible change in pressure per step was set to 20 bar.
NanoFlowSizer for liposome production
A FIDES I type NanoFlowSizer was connected to a LiFT (DIANT Pharma Inc.) liposome manufacturing skid (see Figure 4B). The parameters regarding the PID control of the LiFT system and liposome formulation are property of DIANT Pharma Inc. and are available upon reasonable request.
Acknowledgements
The authors would like to acknowledge the contributions of Eric Ardeche for his help integrating the NanoFlowSizer with the GEA Xstream Lab Homogenizer 1000 HPH and of Dr. Costa for providing the data on liposome production by a LiFT skid (DIANT Pharma Inc.) with an integrated NanoFlowSizer.
References
- Besseling, R., et al. (2019). New unique PAT method and instrument for real-time inline size characterization of concentrated, flowing nanosuspensions. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences, [online] 133, pp.205–213. https://doi.org/10.1016/j.ejps.2019.03.024.
- NewsMedical. (2021). Real-Time Droplet Size Monitoring of Nano Emulsions During High Pressure Homogenization. [online] Available at: https://www.news-medical.net/whitepaper/20241106/Real-time-droplet-size-monitoring-of-nano-emulsions-during-high-pressure-homogenization.aspx
- Costa, A.P., et al. (2015). Liposome Formation Using a Coaxial Turbulent Jet in Co-Flow. Pharmaceutical Research, 33(2), pp.404–416. https://doi.org/10.1007/s11095-015-1798-8.
- Einstein, A. (2004). On the Motion of Small Particles Suspended in Liquids at Rest Required by the Molecular-Kinetic Theory of Heat ∗. [online] Semantic Scholar. Available at: https://www.semanticscholar.org/paper/On-the-Motion-of-Small-Particles-Suspended-in-at-by-Einstein/9c1d91a9f0a37e578ee9a6605b224ad554ec6e86.
- Goodarzi, F. and Zendehboudi, S. (2018). A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. The Canadian Journal of Chemical Engineering, 97(1), pp.281–309. https://doi.org/10.1002/cjce.23336.
- Gupta, A., et al. (2016). Controlling and predicting droplet size of nanoemulsions: scaling relations with experimental validation. Soft Matter, [online] 12(5), pp.1452–1458. https://doi.org/10.1039/C5SM02051D.
- Håkansson, A. (2019). Emulsion Formation by Homogenization: Current Understanding and Future Perspectives. Annual Review of Food Science and Technology, 10(1), pp.239–258. https://doi.org/10.1146/annurev-food-032818-121501.
- AZoNano (2022). AZoNano. [online] AZoNano. Available at: https://www.azonano.com/.
- Kalkman, J., Sprik, R. and van Leeuwen, T.G. (2010). Path-Length-Resolved Diffusive Particle Dynamics in Spectral-Domain Optical Coherence Tomography. Physical Review Letters, 105(19). doi:https://doi.org/10.1103/physrevlett.105.198302.
- ResearchGate. (2023). Available at: https://www.researchgate.net/publication/373990661_Instrument_dependence_of_DLS_particle_size_data_implications_for_data_interpretation_and_standards_measurements.
- Schuurmans, C.C.L.,et al (2022). Inline particle sizing in flow for demanding nanosuspension processes. [online] https://doi.org/10.13140/RG.2.2.18231.80805.
- Wikipedia Contributors (2023). Proportional–integral–derivative controller. [online] Wikipedia. Available at: https://en.wikipedia.org/wiki/Proportional%E2%80%93integral%E2%80%93derivative_controller.
About InProcess-LSP
InProcess-LSP, headquartered in Oss at Pivot Park, is a rapidly growing, innovative company founded in 2014. Backed by a team of in-house experts—comprising physicists, chemists, and software engineers—InProcess-LSP is at the forefront of nanotechnology solutions. The company’s leading product, the NanoFlowSizer, is a cutting-edge instrument designed to deliver inline, real-time measurements of nanoparticles in solution, making it indispensable across various industries.
Utilizing Spatially Resolved Dynamic Light Scattering (SR-DLS) technology, the NanoFlowSizer enables accurate characterization of nanoparticles in flowing liquids, providing critical data such as hydrodynamic diameter, polydispersity index (PDI), and D90 within seconds.
This state-of-the-art instrument empowers both scientists and industries by offering a robust solution for analyzing nanoparticle properties, paving the way for breakthroughs in product development, improved formulations, and pioneering applications.
Innovators in process analytical technology and nanoparticle characterization.
With their strong background in process analytics as well as many years of academic and industrial experience InProcess offer a highly skilled and experienced team of scientists and process specialists addressing the needs of your PAT and nanotechnology challenges.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.