Healthcare environments require effective decontamination procedures. These areas differ in layout, heating, ventilation, air conditioning (HVAC), engineering controls, and room classification (for cleanrooms).
Hospital pharmacies are noteworthy spaces with aseptic conditions requiring maintenance, particularly the cleanrooms developed for managing compounded sterile preparations.
This article explores, implements, and confirms a decontamination protocol for mobile cleanroom trailers. These mobile pharmacies are held in 53 ft (16 m) trailers that meet all USP and cGMP requirements.
The mobile units have combined HVAC systems and must run efficiently in various external environments. Effective sterilization protocols for each trailer deployed are crucial.
A transportable solution is needed to ensure a 6-log sporicidal kill throughout all trailer surfaces. The process must also confirm the eradication of mold and Clostridium difficile, which are spores and pathogens in humans and can be difficult to guarantee a kill.
Another incentive for improving sterilization procedures is to moderate the potential for positive hits for mold.
Background
Multiple verified methods achieve low-temperature sterilization of a pharmacy cleanroom space, including:
- Formaldehyde
- Chlorine dioxide gas
- Vaporized hydrogen peroxide (VHP)
Transporting sterilization equipment, the time required to complete mandatory operations, and the facility’s remote location must be considered due to the unique demands of managing a mobile cleanroom.
Formaldehyde is problematic for remote use as it has a longer contact time than other methods to ensure the required kill. Formaldehyde also needs neutralizing following decontamination. The extra time the team needed demonstrated that this approach was not a viable solution.
In recent years, chlorine dioxide and VHP have become prominent in sterilization. VHP has been more recognized due to its faster “kill time” at the OSHA limit for 8 hours of exposure at 1 ppm.
Chlorine dioxide necessitates a 0.1 ppm concentration for safety, and its higher exposure limit also indicates faster aeration. High-temperature sterilization methods exist, but this is impractical for any room-sized space due to the large amount of solution required. A further consideration is the equipment’s heat sensitivity within the trailer.
VHP is a broadly utilized sterilant for laboratory and cleanroom applications. It is a 35 % v/v mixture of HP and water, a much higher concentration than the 3 % solution in a typical drugstore.
This high-concentration VHP solution also comes with issues. The U.S. Department of Transportation forbids conveying hydrogen peroxide at concentrations above 8 % without a specific license.
The EPA also limits the quantity of high-concentration HP a facility can store and has stringent regulations on how it must be disposed of. Several companies state they can accomplish a kill with HP solutions under 8 %.
One company employed a misting method to disperse the HP within the trailer. The primary results demonstrated that this technique could not achieve the kill required.
A process was then discovered that utilized an ionized HP (iHP) solution developed by TOMI Environmental Solutions. This involved spraying a HP mist through an electrical arc. The electricity changes the HP chemistry to achieve a kill quicker than traditional VHP.
Less contact time allows the HP solution to have a lower concentration. The system was also developed with portability in mind. These considerations created a desirable approach to decontamination for mobile cleanrooms.
The primary issue was that the industry had not fully embraced the iHP process as a validated sterilization method. Germfree completed a validation study to ensure the method could achieve the desired kill.
Proposed solution
The iHP solution was initially tested using a Germfree bioGO™ Rx Mobile Compounding Pharmacy. A satisfactory kill was achieved, but some residue remained following the test’s conclusion.
The protocol was adjusted following a consultation with the decontamination manufacturer, and no residue remained after the second cycle development run. Engineering-level tests began following proper system setup for system validation.
Three identical tests must be run for the system to be considered validated. Around 40 chemical indicators (CIs) and 40 biological indicators (BIs) inoculated with 106 Geobacillus stearothermophilus were positioned throughout the trailer, including the area over the ceiling grid (Figure 1).
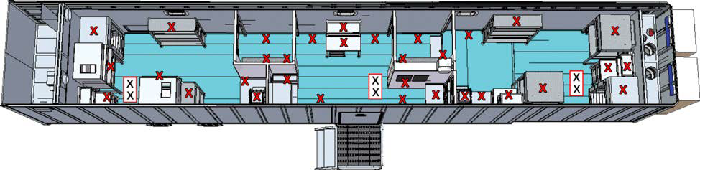
Figure 1. Location of BIs throughout the trailer (boxed X’s were above ceiling panels). Note: The cleanroom trailer was run under normal operational conditions during the testing. The set points are 18.9 C and less than 60% RH. (55% RH is the typical RH at 18.9 °C). Image Credit: SteraMist Disinfection and Decontamination Technology
Geobacillus stearothermophilus is utilized because of its high VHP sterilization resistance comparable to mold and C. Diff. It is non-pathogenic and incubates at reasonably high temperatures (55–60 °C).
The higher incubation temperature enables flexibility on how BI is handled following sterilization, as many bacteria cannot survive at this temperature.
The CIs are paper strips with a dye that changes color upon responding to airborne HP. This delivers a prompt response regarding how the HP is circulated throughout the trailer, but the CIs cannot be utilized to confirm that the system has accomplished a six-log kill.
Once the iHP cycle was finished and the concentration in the trailer was under 1 ppm, the BIs were gathered and aseptically transported to a culture medium—a vial of liquid with a soy protein mixture and a pH indicator inside. If bacteria growth is positive during incubation, the media’s pH level changes, altering the media color from purple to yellow.
Positive and negative controls were incubated alongside the exposed BIs to confirm material validity. The positive control is a BI unexposed to sterilant and put into a media vial. The negative control is an empty media vial.
Each vial is labeled with the date, the test number, and the location number. The location relates to a preestablished map of the BIs. Following FDA guidelines, the BIs and media undergo a 7-day incubation period. Initial results are available following 24 hours of incubation.
Conclusion
All test BIs showed no signs of growth following 7 days of incubation. This proves that the iHP procedure is effective in pharmacy trailer systems. The procedure used multiple BIs in hard-to-reach areas.
Consistent placement of BIs for each of the three validation cycles demonstrated repeatability in this sterilization technique. The solution is under 8 % HP v/v, and the trailers could be serviced in multiple US locations without transportation restrictions.
Another advantage is the system’s design, which comes in a compact, rugged case. Due to its convenience, effectiveness, and more, it is believed to be the preferred sterilization method of Germ-free bioGO Rx Mobile Compounding Pharmacies.
This approach should also be considered for mobile, modular, and fixed laboratories and cleanrooms.
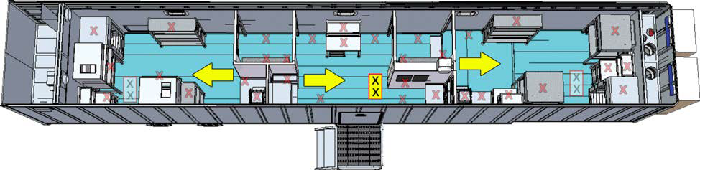
Figure 2. Yellow rectangle shows location of the tile, arrows represent the location of the distribution heads and direction of spray. Image Credit: SteraMist Disinfection and Decontamination Technology
About SteraMist Disinfection and Decontamination Technology
SteraMist is a global line of powerful disinfection and decontamination products and services that utilizes patented ionized Hydrogen Peroxide (iHP) technology.
Our scientific expertise, innovation, and dedication to creating a safer world through cutting-edge iHP technology allow SteraMist to serve our customers and industries with trusted products, leading to a smarter way to decontaminate and control infectious diseases.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.