Cell-free DNA (cfDNA) is tiny fragments of genetic material that circulate in the bloodstream. It can indicate the health of both mother and baby in pregnancy tests or provide hints about cancer in adults.
Image Credit: Kateryna Kon/Shutterstock.com
Why automate cfDNA extraction?
A key obstacle in isolating cfDNA is the intricate, labor-intensive manual process required by existing protocols to extract it for downstream processing.
This article introduces a unique automated cfDNA extraction platform, the INSTA NX® Mag24 (Figure 1B), which runs HiMedia Laboratories’ MB586MPF-24 prefilled extraction cartridge (Figure 1A), reducing processing time by 50% while minimizing inter-sample variability.
Another factor that ensures success in the diagnostic process is the lack of genomic DNA in the extracted eluate.
Temporal study on peripheral blood from pregnant women
Because non-invasive prenatal surveillance is performed at various stages of pregnancy, DNA production from a peripheral blood sample two and seven months after conception was examined. The automated system was compared to manual kits such as the QIAamp Circulating Nucleic Acid Kit.
Consistent DNA output compared to manual extraction
The concentration of cfDNA collected during the second month of pregnancy was 0.36 + 0.06 ng/µL (mean + sd, n=4), which was greater than the industry standard QIAamp Circulating Nucleic Acid Kit (0.169 + 0.05 ng/µL, mean + sd, n=4).
During the seventh month of pregnancy, the automated system yielded 0.24 + 0.06 ng/µL more than the manual QIAamp extraction of 0.22 + 0.02 ng/µL (mean + sd, n = 4).
Isolated cfDNA fragment profile reveals lower co-isolation of gDNA
A comparison of the extract's tapestation 4200 (Agilent) profile revealed a more noticeable genomic DNA peak, resulting in a ~5% reduction in total cfDNA isolated using the QIAamp Circulating Nucleic Acid Kit.
Successful NGS library preparation and sequencing on Illumina NextSeq550 platform
A hybrid capture-based Illumina library preparation resulted in all called bases having a Q value above 30 (Figure 3), with around 75% of the reads acquired being unique across both kits.
Field trial further shows consistent extraction and sequencing on non-Illumina platform
A field trial of the kit was conducted in a clinical setting with more than 300 samples. The automated extractor's yield consistently matched that of the QIAmp Circulating Nucleic Acid kit.
The generated cfDNA was proven compatible with the Yourgene QS250 size selection process and Ion Chef Library prep automation and provided outstanding results on the Ion Genestudio S5 system, which is commonly used for non-invasive prenatal testing.
Conclusion
In conclusion, this study found that the automated cfDNA extraction platform INSTA NX® Mag24 with an MB586MPF-24 prefilled extraction cartridge produces effective and uniform cfDNA extraction compatible with various second-generation sequencing platforms. It can also offer consistency identical to that of manual extraction methods.
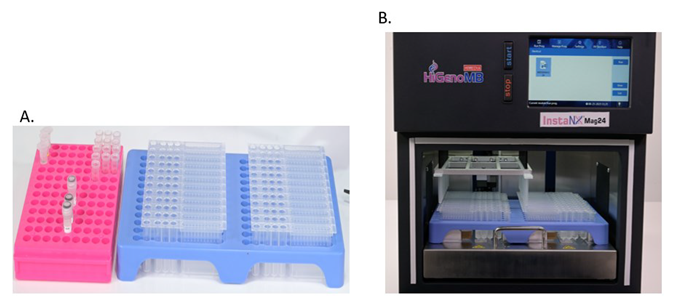
Figure 1. Components of (A) MB586MPF-24 prefilled cartridge. (B) Arrangement of the consumables on the automated cfDNA extraction platform INSTA NX® Mag24 machine. Image Credit: Himedia Laboratories Private Limited
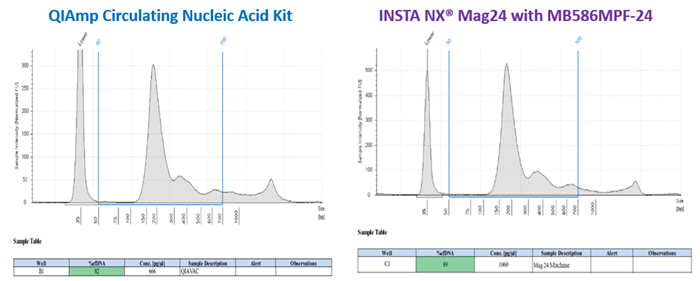
Figure 2. cfDNA profile as observed in an Agilent™ tape station 4200. A more pronounced genomic DNA peak is observed in case of QIAmp CNA kit relative to the automated system with greater % cfDNA yield. Image Credit: Himedia Laboratories Private Limited
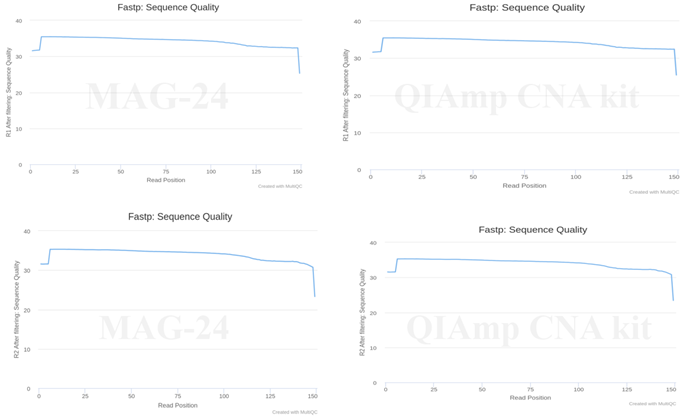
Figure 3. Representative data quality was observed using 2x150bp sequencing on Illumina NextSeq550 instrument using a hybridization capture-based library. Image Credit: Himedia Laboratories Private Limited
About Himedia Laboratories Private Limited
With a presence in more than 150 countries, HiMedia is among the top three brands in the Bioscience Industry.
HiMedia Laboratories Private Limited is world-renowned for manufacturing high-quality culture media for microbiology. Additionally, we provide advanced media and products in the fields of Molecular Biology, Cell Biology, Plant Tissue Culture, Chemicals and Lab Aids/Equipment. As a Top Tier Global player, we are not only dedicated toward products but also striven towards introducing technologies such as Genomics Sequencing Services and Hydroponics.
HiMedia has managed to do this over decades as we have our own in-house bulk raw materials manufacturing plant. This enables us to deliver consistent quality products that conform to ISO 9001:2015 and ISO 13485:2012 and WHO: GMP.
HiMedia Labs. caters to one of the broadest Biosciences product categories: our premier established line of Microbiology products and newer promising products in Molecular Biology, Automated and Molecular Instruments, Cell Biology, Chemicals, and Premium Grade Lab Consumables, amongst others. The COVID-19 pandemic revolutionized not the clinical industry’s thought process regarding the significance of Molecular Diagnostics products.
The ‘Molecular Biology and Virology Division’ of HiMedia Laboratories Pvt. Ltd. Also called as HiGenoMB® is a One Stop Solution Provider churning out potential Research and Industry oriented Molecular biology products for the past glorious decade. About 2000 different products such as Nucleic Acid Extraction and Amplification (PCR) Kits, Cloning Reagents, Buffers & Chemicals for proteomics studies, Automated Molecular Instrumentation including RT PCR machines and PCR thermal cyclers and DNA/RNA Extraction platforms are being produced. The Proficient researchers in this department are spear heading the challenging field of Molecular Diagnostics to provide a complete solution for clinical diagnosis, agriculture, veterinary sciences, food industry, drug discovery and forensic medicine with the use of Real Time PCR or quantitative PCR kits and thermal cyclers. Our Molecular Biology Division-has established an in-house Advanced Sequencing and Bioinformatics facility which marks HiMedia’s entry into the Services space.
Our Cell Biology segment contributes with technologies which have brought in Serum free media for biopharma applications, Viral Vaccine Production Platform, Multicompendial grade chemicals, cultivated meat, and 3D bioprinting.
Moving from conventional to advanced automated methods like MALDI-TOF (Autof MS 1000) has been our newest endeavour for Microbiology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.