Fragmented cell-free DNA (cfDNA) molecules were found in the human circulatory system in 1948. Afterward, sequencing of these fragments proved their importance in distinguishing healthy from diseased samples.
Image Credit: Kateryna Kon/Shutterstock.com
However, to fully utilize cfDNA for diagnostic reasons, it must be efficiently isolated from the bloodstream. Magnetic bead-based extraction methods may now extract PCR-amplifiable and sequencing-compatible cfDNA.
HiMedia Laboratories’ Insta NX® Mag24 machine, with a prefilled single sample cartridge, completely automates this extraction process.
One to twenty-four samples can be processed in a single run to isolate free-circulating DNA from fetal-originated DNA in maternal blood or tumor-originating cell-free DNA fragments in blood. Unlike its competitor (the QIAvac system), this automatic system does not require sample pretreatment or manual handling.
Objective
Fifty peripheral blood samples were extracted using the HiPurA® Cell-free DNA Purification Kit compatible with Insta NX® Mag24 system (Magnetic) and then compared to the QIAamp Circulating Nucleic Acid Kit on the QIAvac system (Column).
Method and material
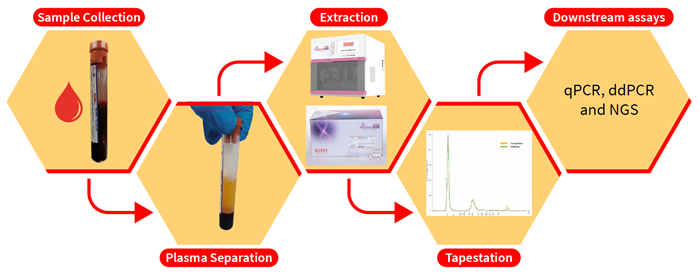
Image Credit: Himedia Laboratories Private Limited
Results
Although cfDNA, extracted with QIAvac, yielded a greater DNA concentration than HiMedia's kit, fragment analysis with Tapestation4200 revealed considerable amounts of genomic DNA, which explains the high DNA concentration.
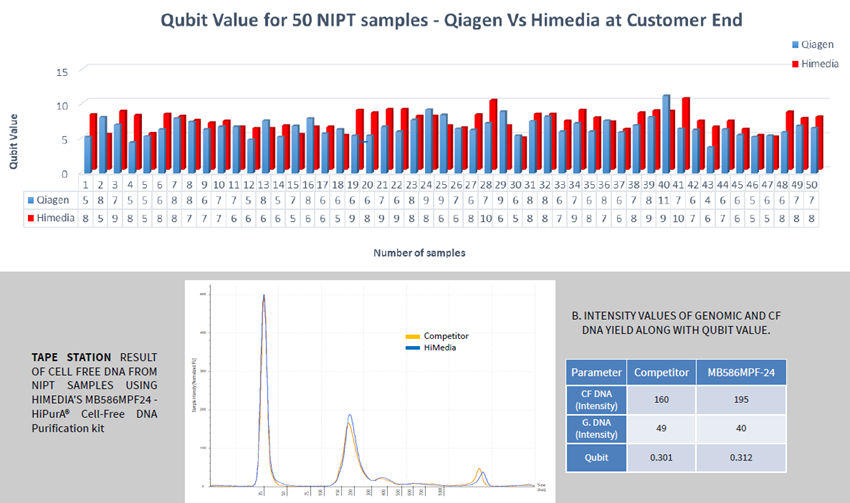
Image Credit: Himedia Laboratories Private Limited
Conclusion
HiPurA® Cell-free DNA Purification Kit, compatible with Insta NX® Mag24, yields higher cfDNA recovery than the QIAamp Circulating Nucleic Acid Kit for the QIAvac system.
Acknowledgments
Produced from materials originally authored by Dipali Rane, Sneha Thakur, Somak Chuadhary, Rajas Warke, and Kavita Khadke at HiMedia Laboratories Pvt. Ltd.
References and further reading
- Zhang, J., et al. (2019). Non-invasive prenatal sequencing for multiple Mendelian monogenic disorders using circulating cell-free fetal DNA. Nature Medicine, [online] 25(3), pp.439–447. https://doi.org/10.1038/s41591-018-0334-x.
- Zhong, H., et al. (2022). A novel method for extracting circulating cell‐free DNA from whole blood samples and its utility in the non‐invasive prenatal test. Prenatal Diagnosis, 42(9), pp.1173–1181. https://doi.org/10.1002/pd.6212.
About Himedia Laboratories Private Limited
With a presence in more than 150 countries, HiMedia is amongst the top three brands in the Bioscience Industry.
HiMedia Laboratories Private Limited is world renowned for manufacturing high quality culture media for microbiology. Additionally, we provide advanced media and products in the fields of Molecular Biology, Cell Biology, Plant Tissue Culture, Chemicals and Lab Aids/Equipment. As a Top Tier Global player, we are not only dedicated towards products but also striven towards introducing technologies such as Genomics Sequencing Services and Hydroponics.
HiMedia has managed to do this over decades as we have our own in-house bulk raw materials manufacturing plant. This enables us to deliver consistent quality products that conform to ISO 9001:2015 and ISO 13485:2012 and WHO: GMP.
HiMedia Labs. caters to one of the broadest Biosciences product categories: our premier established line of Microbiology products and newer promising products in Molecular Biology, Automated and Molecular Instruments, Cell Biology, Chemicals, and Premium Grade Lab Consumables, amongst others. The COVID-19 pandemic revolutionized not the clinical industry’s thought process regarding the significance of Molecular Diagnostics products.
The ‘Molecular Biology and Virology Division’ of HiMedia Laboratories Pvt. Ltd. Also called as HiGenoMB® is a One Stop Solution Provider churning out potential Research and Industry oriented Molecular biology products for the past glorious decade. About 2000 different products such as Nucleic Acid Extraction and Amplification (PCR) Kits, Cloning Reagents, Buffers & Chemicals for proteomics studies, Automated Molecular Instrumentation including RT PCR machines and PCR thermal cyclers and DNA/RNA Extraction platforms are being produced. The Proficient researchers in this department are spear heading the challenging field of Molecular Diagnostics to provide a complete solution for clinical diagnosis, agriculture, veterinary sciences, food industry, drug discovery and forensic medicine with the use of Real Time PCR or quantitative PCR kits and thermal cyclers. Our Molecular Biology Division-has established an in-house Advanced Sequencing and Bioinformatics facility which marks HiMedia’s entry into the Services space.
Our Cell Biology segment contributes with technologies which have brought in Serum free media for biopharma applications, Viral Vaccine Production Platform, Multicompendial grade chemicals, cultivated meat, and 3D bioprinting.
Moving from conventional to advanced automated methods like MALDI-TOF (Autof MS 1000) has been our newest endeavour for Microbiology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.