Acute fever illness (AFI) is characterized by a sudden onset of fever without identifiable organ-specific signs or symptoms. Infectious AFI tends to be more common in tropical and subtropical areas, where the climate supports the survival of transmission vectors like mosquitoes and ticks.
Image Credit: Kateryna Kon/Shutterstock.com
Arthropod-borne diseases such as Dengue, Chikungunya, Malaria (caused by Plasmodium spp.), and Zika are endemic and re-emerging infections in tropical and subtropical regions. During heavy monsoon seasons, Leptospirosis, Scrub typhus (caused by Orientia tsutsugamushi), and Enteric fever (caused by Salmonella spp.) are also highly prevalent.
Physicians face challenges diagnosing these infections due to similar clinical symptoms. Timely diagnosis is critical for effective patient management and averting life-threatening complications. Accurate early diagnosis is essential for treating patients and controlling disease transmission, preventing large outbreaks, and reducing unnecessary antibiotic use.
Hi‐PCR® Acute Fever Panel Kit (MBPCR278) is designed to identify Dengue virus, Chikungunya virus, Zika virus, Salmonella spp., Leptospira spp., Plasmodium spp. and Orientia tsutsugamushi in clinical samples.
Performance characteristics of the Acute fever panel kit
Analytical sensitivity
The Limit of Detection (LoD) of the Hi‐PCR® Acute Fever Panel Kit was tested using 20 replicates each on Biorad CFX Opus 96, Applied Biosystems QuantStudio 5 and Insta Q96® Plus Real Time PCR Systems using Quantitative nucleic acids for Dengue virus
- AMPLIRUN® Dengue virus RNA controls from Vircell Microbiologics, DENV 1 (MBC055‐R), Zika virus.
- Quantitative Genomic RNA from Zika virus strain PRVABC59 (VR‐1843DQ) from ATCC, Salmonella spp
- Salmonella typhi (ATCC: 14028) from ATCC, Leptospira spp.
- Leptospira interrogans serovar Copenhageni strain Fiocruz L1‐130 (ATCC: 1198D‐5) from ATCC and Plasmodium spp. - Plasmodium falciparum strain 3D7 (ATCC: 405D) from ATCC and synthetic nucleic acid for Chikungunya virus and Orientia tsutsugamushi.
The data revealed that the kit detects LoD of each target mentioned in Table 1 with ≥ 95% confidence.
Table 1. Analytical sensitivity of the Hi‐PCR® Acute Fever Panel Kit. Source: Himedia Laboratories Private Limited
| Limit of detection (LoD) |
| Dengue virus |
10 copies/μl |
| Zika virus |
1 copy/ μl |
| Chikungunya virus |
1.87 copies/ μl |
| Salmonella spp. |
1 copy/ μl |
| Leptospira spp. |
1 copy/ μl |
| Plasmodium spp. |
1 copy/ μl |
| Orientia tsutsugamushi |
5 copies/ μl |
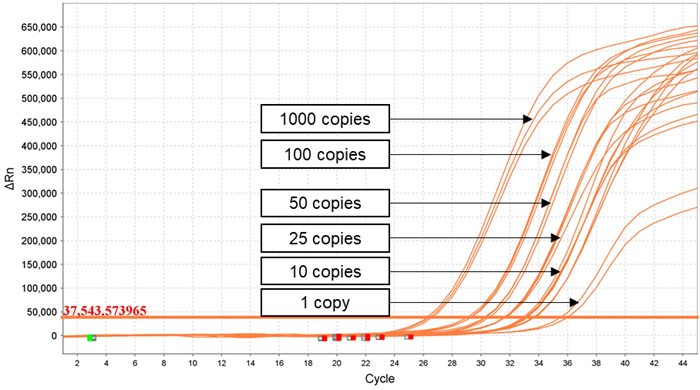
Figure 1. Dilution series of Quantitative Genomic RNA from Zika virus strain PRVABC59 (VR‐1843DQ), ATCC from 1000 genome copies/ μl to 1 genome copy/ μl analyzed using Applied Biosystems QuantStudio 5. Image Credit: Himedia Laboratories Private Limited
Analytical specificity
The Hi‐PCR® Acute Fever Panel Kit has been evaluated for its capability to specifically identify a broad spectrum of related target organisms while differentiating them from genetically distinct non-target organisms.
This assessment was conducted through two approaches: (i) an in silico analysis of the oligonucleotides (primers and probes) and (ii) laboratory testing using nucleic acids from related target organisms, including members of the Flavivirus family, which are linked to viral hemorrhagic fevers and are responsible for mosquito-borne diseases that can lead to acute febrile illness.
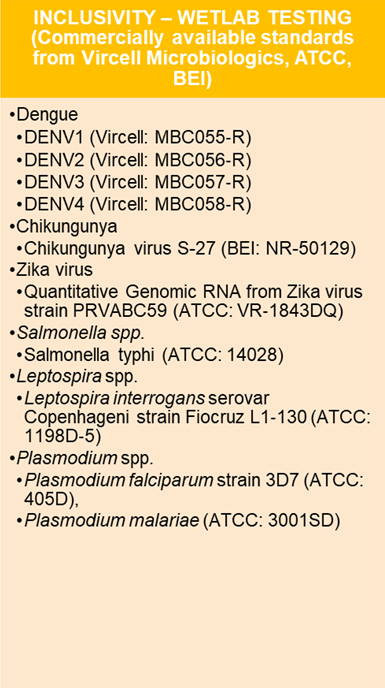
Image Credit: Himedia Laboratories Private Limited

Image Credit: Himedia Laboratories Private Limited
Precision studies
The precision of the Hi‐PCR® Acute Fever Panel Kit (MBPCR278) was evaluated under various conditions, including:
- Inter-day variability: Variability measured across three different days.
- Inter-operator variability: Variability assessed among three different operators.
- Inter-instrument variability: Variability analyzed using three different PCR thermal cyclers.
- Inter-lot variability: Variability compared between three different lots of the kit.
The variability data were analyzed in terms of standard deviation (SD) and percent coefficient of variation (CV) based on threshold cycle (Ct) values. Results from all four variability analyses indicated that the percent CV was less than 5% across all tested targets.
Table 2. Precision studies of Hi‐PCR® Acute Fever Panel Kit (MBPCR278). Source: Himedia Laboratories Private Limited
| |
Inter-day variability |
Inter-operator variability |
Inter-Machine variability |
Inter- Lot variability |
| Targets |
Mean Ct |
SD |
%CV |
Mean Ct |
SD |
%CV |
Mean Ct |
SD |
%CV |
Mean Ct |
SD |
%CV |
| Chikungunya |
24.68 |
0.22 |
0.89 |
24.27 |
0.48 |
1.97 |
23.93 |
0.58 |
2.41 |
24.55 |
0.36 |
1.48 |
| Dengue |
26.31 |
0.38 |
1.44 |
25.70 |
0.58 |
2.24 |
25.67 |
0.17 |
0.67 |
26.04 |
0.51 |
1.94 |
| L35A |
28.16 |
0.14 |
0.49 |
27.94 |
0.26 |
0.94 |
28.60 |
0.51 |
1.80 |
28.32 |
0.18 |
0.65 |
| Zika |
26.34 |
0.13 |
0.48 |
26.16 |
0.26 |
0.99 |
27.77 |
1.35 |
4.85 |
26.28 |
0.18 |
0.68 |
| Leptospira |
23.12 |
0.17 |
0.74 |
23.05 |
0.29 |
1.27 |
22.06 |
1.16 |
5.25 |
23.10 |
0.31 |
1.34 |
| Plasmodium |
22.66 |
0.14 |
0.62 |
22.68 |
0.08 |
0.35 |
22.78 |
0.52 |
2.28 |
22.79 |
0.23 |
0.99 |
| Salmonella |
19.59 |
0.13 |
0.68 |
19.53 |
0.09 |
0.47 |
20.37 |
0.41 |
2.03 |
19.74 |
0.25 |
1.25 |
| Orientia tsutsugamushi |
23.40 |
0.16 |
0.68 |
23.36 |
0.13 |
0.54 |
23.21 |
0.51 |
2.22 |
23.63 |
0.20 |
0.84 |
Conclusions
Multiplex real-time PCR-based diagnostic tests facilitate the simultaneous detection of multiple pathogens in a single assay, thereby conserving resources and time. The Hi‐PCR® Acute Fever Panel Kit (MBPCR278) provides a sensitive and specific solution for diagnosing infectious acute fever illnesses.
About Himedia Laboratories Private Limited
With a presence in more than 150 countries, HiMedia is amongst the top three brands in the Bioscience Industry.
HiMedia Laboratories Private Limited is world renowned for manufacturing high quality culture media for microbiology. Additionally, we provide advanced media and products in the fields of Molecular Biology, Cell Biology, Plant Tissue Culture, Chemicals and Lab Aids/Equipment. As a Top Tier Global player, we are not only dedicated towards products but also striven towards introducing technologies such as Genomics Sequencing Services and Hydroponics.
HiMedia has managed to do this over decades as we have our own in-house bulk raw materials manufacturing plant. This enables us to deliver consistent quality products that conform to ISO 9001:2015 and ISO 13485:2012 and WHO: GMP.
HiMedia Labs. caters to one of the broadest Biosciences product categories: our premier established line of Microbiology products and newer promising products in Molecular Biology, Automated and Molecular Instruments, Cell Biology, Chemicals, and Premium Grade Lab Consumables, amongst others. The COVID-19 pandemic revolutionized not the clinical industry’s thought process regarding the significance of Molecular Diagnostics products.
The ‘Molecular Biology and Virology Division’ of HiMedia Laboratories Pvt. Ltd. Also called as HiGenoMB® is a One Stop Solution Provider churning out potential Research and Industry oriented Molecular biology products for the past glorious decade. About 2000 different products such as Nucleic Acid Extraction and Amplification (PCR) Kits, Cloning Reagents, Buffers & Chemicals for proteomics studies, Automated Molecular Instrumentation including RT PCR machines and PCR thermal cyclers and DNA/RNA Extraction platforms are being produced. The Proficient researchers in this department are spear heading the challenging field of Molecular Diagnostics to provide a complete solution for clinical diagnosis, agriculture, veterinary sciences, food industry, drug discovery and forensic medicine with the use of Real Time PCR or quantitative PCR kits and thermal cyclers. Our Molecular Biology Division-has established an in-house Advanced Sequencing and Bioinformatics facility which marks HiMedia’s entry into the Services space.
Our Cell Biology segment contributes with technologies which have brought in Serum free media for biopharma applications, Viral Vaccine Production Platform, Multicompendial grade chemicals, cultivated meat, and 3D bioprinting.
Moving from conventional to advanced automated methods like MALDI-TOF (Autof MS 1000) has been our newest endeavour for Microbiology.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.