Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are a frequently used medication all around the world due to their availability as both prescription-only medicines and as over-the-counter preparations.
Image Credit:Shutterstock/Kateryna Kon
Yet, low dose use of NSAIDS is also related to gastrointestinal (GI) injury. Schemes to avoid GI complications, including those linked with NSAID use, are usually associated with unwanted side-effects.
Live bacteria formulated as probiotics may provide a safe alternative to prevent or at least reduce the negative side effects of NSAIDs. The current clinical trial is hoping to bring a product that contains a probiotic strain able to treat and/or reverse NSAIDs-induced small intestinal damage and GI symptoms in NSAIDs users.
Probiotic bacteria are known for the potential to deliver therapeutic effects against intestinal inflammation. The sponsor, a company that is a world-leader in innovation, is determined to bring a treatment to the market, having previously performed numerous in vitro screening assays in order to characterize around 200 different strains.
Five strains were then chosen based on their in vitro characteristics and subsequently tested in a rat model of colitis.
The clinical trial was the first in a clinical development program pursuing a product that carries the selected strain with the ability to treat and/or reverse NSAIDs-induced small intestinal damage and GI symptoms in NSAIDs users.
The product under investigation, per daily dose vegetable capsules containing the probiotics, was completely manufactured by the sponsor in the same batch. The sponsor also holds the necessary certification for food production.
Challenges and objectives
The sponsor required a research partner with extensive experience and the ability to assess the deterioration of small intestinal mucosa tissue. However, they needed a technique that was not overly invasive in order to overcome any ethical burdens. The primary objectives were:
- To study the probiotic strain and determine its capacity to treat and/or reverse low dose, long-term NSAIDs-induced deterioration of small intestinal mucosa tissue evaluated and administered via capsule endoscopy in healthy volunteers.
- To examine the ability of the probiotic strain to treat and reverse low dose, long term NSAIDs-induced GI symptoms as assessed AUC ulcer number as well as assessed by AUC of pain syndrome score for GSRS.
- To evaluate co-administration of the probiotic strain to low dose, long term NSAIDs on changes across several biomarkers of typical intestinal barrier function in blood and fecal samples.
How Atlantia’s solution helped
Consequently, a clinical challenge model aiming at evaluating the probiotic strain’s capacity in treating and/or reversing deterioration in the healthy human gastrointestinal tract was established.
The deterioration was initiated by a frequently used chemical agent known for its deteriorating effects on the small intestine. For the primary endpoint, method capsule endoscopy (CE) was utilized to assess the damage to the small intestinal.
Reviewed in a technology status evaluation report by the American Society for Gastrointestinal Endoscopy, capsule endoscopy is now the baseline for assessing occult gastrointestinal bleeding, and applications for its use are continually expanding.
Existing uses include exploration and observation of bowel pathologies such as in Crohn’s disease, small bowel malignancy, polyps and drug-induced mucosal injury.
Capsule endoscopy (CE) is typically regarded as a safe and well-tolerated procedure. Atlantia was a perfect fit for the sponsor due to its in-house team, which possesses great expertise in managing these technologies when conducting trials.
About the study
The trial was a single-site, randomized, two-armed, placebo-controlled, double-blind, parallel group trial in healthy, adult volunteers. Evaluating the effects of a daily intake of the probiotic strain or placebo when coadministered with 300 mg of Aspirin.
The trial was carried out in-line with the ethical principles determined by the current versions of the Declaration of Helsinki (seventh revision; October 2013), the International Conference on Harmonization E6 Good Clinical Practice (ICH-GCP, 10 June 1996) and all relative local regulatory requirements.
The trial was made up of a run-in period of two weeks followed up by a six-week intervention period coadministering the probiotic/placebo and NSAIDs. After the six weeks, the probiotic/placebo was provided for a further two weeks to evaluate the possible effects of the probiotic on intestinal healing after long-term NSAIDs use.
Total subject participation in the trial spanned ten weeks, including the run-in phase.
The principal efficacy variable was an examination of the effect of oral supplement of the probiotic strain in contrast to the placebo on small intestinal mucosa damage when coadministered with NSAIDs - the challenge was measured as the area-under-the-curve (AUC) for Lewis Score acquired by capsule endoscopy.
The sample size in each arm was 30 completing participants, for which an approximate power calculation was carried out at intervention based on the percentage difference of AUC between two normalized curves (active vs. placebo).
Source: Atlantia Clinical Trials
| Recruitment |
Number of subjects |
| Planned |
75 |
| Screened |
140 |
| Randomized |
75 |
| Dropouts |
17 |
| Completed |
66 |
Taking into account a potential drop-out rate of approximately 15%, in each group, a total of 35 subjects were randomized. Following treatment with the probiotic versus placebo, a significant clinical decrease was observed in the AUC data.
During the entire trial, subjects were instructed to maintain their normal life-style where diet, physical activity level and sleep habits were considered. Intake food and food supplements containing probiotics and other probiotic products were prohibited from the screening visit until the end of the intervention period.
Yet, single violations did not lead to any subjects being withdrawn from the trial, but violations were recorded as protocol deviations.
Small intestine mucosa deterioration was assessed utilizing video capsule endoscopy as well as indirect biomarkers in blood samples and feces. During their visit, each subject completed the GSRS questionnaire, which evaluated GI symptoms and pain.
Each subject experienced a video capsule endoscopy during the 8-week intervention. Capsule endoscopy was deemed to be an appropriate method to assess both mild and severe intestinal damage caused by a low dosage of Aspirin measured as area-under-the-curve.
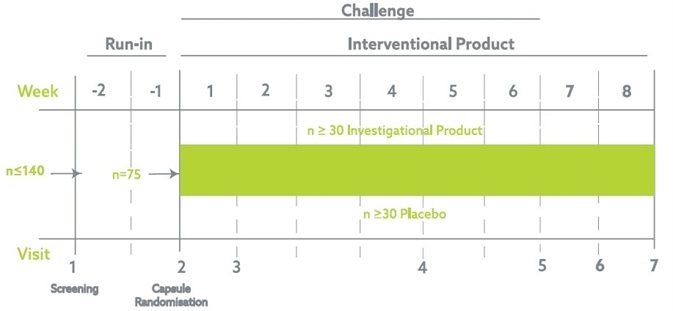
Image Credit: Atlantia Clinical Trials
Future plans and return on investment
The findings of the study demonstrate that capsule endoscopy is a strong and reliable method for measuring intestinal damage. The GSRS questionnaire identified a response in some of the categories, but the challenge signal was generally very small, and the data showed no effect of the intervention.
While blood I-FABP and fecal calprotectin responded to the NSAIDs challenge, VCE should continue to be the method preferred above all of the aforementioned clinical activities, biomarkers, and/or questionnaires.
The AUC approach facilitated the requisite sensitivity to observe intervention effects and should be repeated in future clinical activities relative to this project. Further studies are needed to establish if probiotics can aid the reversal of GI damage in the period after NSAIDs intake (recovery).
A number of significant statistical findings in exploratory endpoints related to ulcers further support the clinical development of the probiotic selected.
This clinical trial clearly shows that subjects who were selected at random to receive the probiotic responded considerably better to the Aspirin challenge model in relation to the primary outcome measure, as well as several of the secondary and exploratory outcomes related to ulcers.
The dataset experienced minimal protocol violations making it excellent and robust where product accountability is concerned. After completion, this study was published in a high impact journal.
About Atlantia Clinical Trials
Atlantia Clinical Trials Ltd is a CRO that specializes in conducting studies in foods, beverages and supplements for companies world-wide that want to scientifically validate their functional ingredients to support an: EFSA (European Food Safety Authority) Health Claim; FDA (Food & Drug Administration) Structure Function Claim; or General Product Marketing Claim.
Atlantia works with world leading scientists (among the top cited 1% internationally, in the areas of digestive health and functional foods) at the: APC Microbiome Institute in University College Cork, Ireland; Teagasc, Moorepark, Ireland and recognized centers of excellence globally.
Atlantia runs and operates its own clinic sites and conducts all studies to ICH-GCP standard (International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use - Good Clinical Practice). Its team includes physician experts in digestive health, mental health (psychological stress and cognition), cardiovascular health, sports performance, metabolic disease, bone health, immune health and healthy ageing. The clinical team also includes project managers, research nurses, nutritionists, certified sports trainers and lab researchers.
Atlantia manages all elements from protocol design, placebo manufacture, recruitment, and study execution, to sample and data analysis, statistics and report/dossier preparation to provide a service which is technically, scientifically and clinically superior.
The clinical studies cover a broad spectrum of functional food and beverage categories, such as dairy, cereal, probiotic, different protein forms, infant-specific foods, vitamins/minerals, plant or marine extracts and medical foods.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.