Atlantia Clinical Trials first conducted a pilot study to evaluate the carryover effect and establish the washout period necessary for the randomized, double-blind, placebo-controlled crossover study and deliver preliminary data on the subject characteristics and variation in the measured parameters.
Irritable bowel syndrome (IBS) typically pertains to chronic abdominal pain, bloating, discomfort and alteration of bowel habits and affects 10-15% of the general population.
In a recent study, Atlantia used two well-characterized strains of probiotic, each of which has interesting properties related to IBS. The first one has demonstrated the capacity to attenuate anxiety and stress and improve cognition in preclinical and clinical studies.
The second strain has been previously subjected to extensive research and shown to regulate inflammatory responses and minimize abdominal pain, bloating, gas and unpredictable bowel habits in IBS subjects in two well-managed clinical studies.
Therefore, the objective of a recent trial was to assess the effect a mixture of both strains has on bowel symptoms and stress/mood in adults with IBS. The research project was comprised of two phases: a pilot study and a randomized, double-blind, placebo controlled crossover study.
Challenges and objectives
The main goal was to evaluate the product under investigation, and its influence on stress and mood in adults with IBS based on change in HADs score.
Other objectives included assessing the effect of the product on IBS symptoms, abdominal pain/discomfort and individual symptoms, such as abdominal bloating/distension, abdominal pain/ discomfort, bowel movement urgency, stool frequency, stool consistency (Bristol Stool Scale), straining and passage of gas.
During the study, the safety of the investigational product was assessed on the basis of serious and non-serious adverse events and safety parameters. The greatest challenge was to check that an interruption from one period did not have a residuary effect that carried over into the following period in a cross-over design.
This is known as a carryover effect. Therefore, a technical team with knowledge of these designs was required.
How Atlantia’s solution helped
Atlantia’s leading team of doctors, scientists, nutritionists and research nurses collaborated with the sponsors’ researchers to design and deliver the primary objectives of the study.
The in-house team of experts that specialize in stress and anxiety was a complete match for the research requirements of the sponsor, and Atlantia’s team of experts recommended a crossover study design.
Each participant acted as their control, and both interventions (the investigational product and placebo) were evaluated for the same individual. This facilitated a trial with a precision on par with a parallel group trial using only half the sample size.
Image Credit: Atlantia Clinical Trials
About the study
The underlying factors contributing to IBS are heterogeneous and not fully understood, but it is believed that alterations in gut mucosal immune activation cognitive function, increased psychological stress, brain-gut interaction and changes in the gut microbiota all contribute significantly to the condition.
Atlantia initially conducted a pilot study to assess the carryover effect and establish the washout period necessary for the randomized, double-blind, placebo-controlled crossover study and supply preliminary data on the subject characteristics and variation in the parameters measured.
In both the pilot and crossover study, stress and mood were measured using endorsed questionnaires and other biological biomarkers.
Bowel symptoms were evaluated using self-reporting methods that accounted for abdominal pain, passage of gas, stool form and consistency, as well as straining and bowel movement urgency on a daily basis using an eDiary system. Other measures include weekly global evaluation of IBS symptoms and abdominal pain/discomfort.
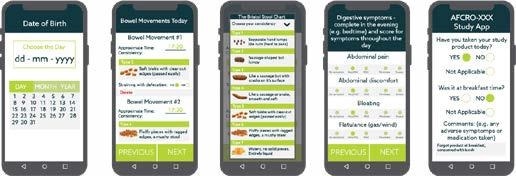
Image Credit: Atlantia Clinical Trials
Atlantia recruited subjects through its database, the offices of general practitioners and adverts posted in local newspapers.
Subjects were initially screened over the phone, answering questions regarding their age, history of mood and stress, gastrointestinal symptoms and previous and current medications. Subjects that were deemed eligible were subsequently scheduled for a face-to-face screening visit.
The study was carried as detailed in the protocol and in line with the ICH Guidelines on Good Clinical Practice and the Declaration of Helsinki.
The pilot study was an open label design as detailed below.
The volunteers all took the investigational product for 8 weeks, followed by an 8-week check-up.
Volunteers were screened in accordance with the selection criteria. Subjects were screened to determine and draw from a pool of participants between the age of 18 and 55 with repeated abdominal pain/discomfort and mild to moderate stress/mood status.
The study necessitated visits over a 20-week period: during each visit, a blood sample was collected. A urine sample was taken at the screening and baseline visits, and upon completion of the intervention. For women of childbearing age, a pregnancy test was carried out.
On each morning of a visit, subjects were advised to avoid brushing their teeth or using mouth wash as a saliva sample was collected on the morning of each visit. Subjects were given a stool collection kit at visits and advised to collect a sample at home and bring it to the clinic for each subsequent visit.
Stool samples were stored at –80 ºC and analyzed for presence/absence of the probiotic strains as an indication of transit/washout of the probiotic strains and saved for compositional analysis, microbiota diversity analysis, SCFA analysis and metabolomics.
The second study was a randomized, double blinded, placebo-controlled study using a repeated measures cross-over design as described.
Volunteers were screened in accordance with the selection criteria. Participants were between 18 and 55 years of age with IBS suffering from recurrent abdominal pain/discomfort and mild to moderate stress/mood status.
The study necessitated periodic visits over 28 weeks. The volunteers were randomized into Group 1 or Group 2. They then went through an 8-week intervention with either the investigational product or matching placebo with a washout period between interventions.
Furthermore, urine, saliva, blood and fecal samples were collected during the crossover study to evaluate endpoints similar to those in the pilot study.
About Atlantia Clinical Trials
Atlantia Clinical Trials Ltd is a CRO that specializes in conducting studies in foods, beverages and supplements for companies world-wide that want to scientifically validate their functional ingredients to support an: EFSA (European Food Safety Authority) Health Claim; FDA (Food & Drug Administration) Structure Function Claim; or General Product Marketing Claim.
Atlantia works with world leading scientists (among the top cited 1% internationally, in the areas of digestive health and functional foods) at the: APC Microbiome Institute in University College Cork, Ireland; Teagasc, Moorepark, Ireland and recognized centers of excellence globally.
Atlantia runs and operates its own clinic sites and conducts all studies to ICH-GCP standard (International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use - Good Clinical Practice). Its team includes physician experts in digestive health, mental health (psychological stress and cognition), cardiovascular health, sports performance, metabolic disease, bone health, immune health and healthy ageing. The clinical team also includes project managers, research nurses, nutritionists, certified sports trainers and lab researchers.
Atlantia manages all elements from protocol design, placebo manufacture, recruitment, and study execution, to sample and data analysis, statistics and report/dossier preparation to provide a service which is technically, scientifically and clinically superior.
The clinical studies cover a broad spectrum of functional food and beverage categories, such as dairy, cereal, probiotic, different protein forms, infant-specific foods, vitamins/minerals, plant or marine extracts and medical foods.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.