Image Credit:Shutterstock/urfin
Currently, visceral adipose tissue accumulation is a key research issue due to its relation to metabolic disturbances, type 2 diabetes and cardiovascular disease.
This study highlights how Atlantia developed and performed a study evaluating the outcome of probiotics supplementation in body composition and weight management reviewed using DXA scans.
About the sponsor
The sponsor is a pioneering and trusted manufacturer of premium probiotics. Their in-house research teams are dedicated to formulating probiotics that endorse the desired structure-function claims, but they required a partner that could develop and deliver a trial with gold standard status.
The sponsor company has previously held an internal trial; however, the study was not appropriate for reaching any statistical significance across groups in several key areas.
Challenges and objectives
The grounds and reason for the latest evaluation are to conduct a controlled follow-up and randomized trial to continue to acquire the necessary evidence for this probiotic strain in the area of weight management.
The sponsors required a trusted partner with the ability to access the required study population, with sufficient expertise to develop an optimized probiotic trial set out to achieve the following objectives:
- To identify the effect of daily administration of a probiotic strain on Visceral Adipose Tissue (VAT), as well as on other efficacy parameters and secondary biomarkers.
- To establish the effect of a daily administration of a probiotic strain on the characteristics of fecal matter.
How Atlantia’s solution helped
Atlantia’s world beating team of researchers and nutritionists worked in close alliance with the sponsors’ researchers to develop and deliver the objectives of the study. In Europe, Ireland has one of the highest rates of obesity.
The percentage of people who were categorized as obese or overweight in 2015 was 60% (Irish Health Survey), which has since risen to 62% in 2017 (CSO).
The Cork-based research clinic Atlantia owns and operates determined to be a prime location to recruit subjects classified as overweight within the sponsor's expected timeline. The company’s expertise in measuring VAT by DXA was also a critical factor for the basis of this project.
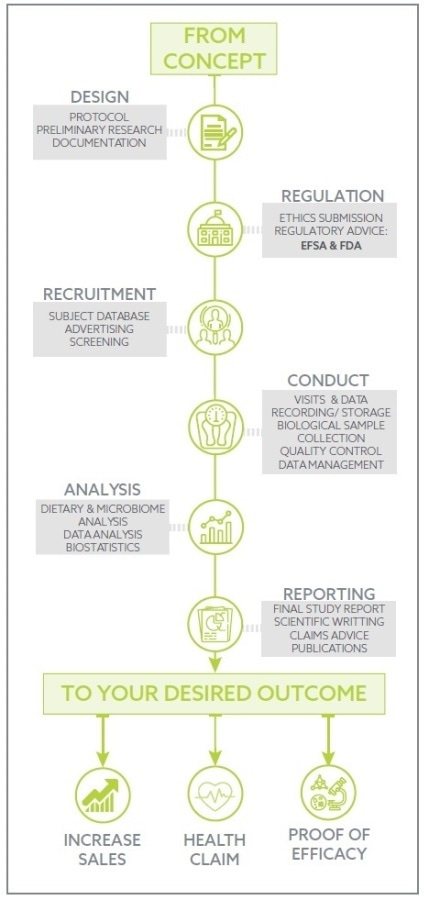
Image Credit: Atlantia Clinical Trials
About the study
This study was a single-center, double-blind, placebo-controlled, randomized, parallel study in overweight volunteers. The total number of participants was 126 with an age range between 25 and 65 years old and a body mass index between 25.0 to 29.9 Kg/m2.
Other parameters included a waist-hip ratio of ≥0.91 for males and ≥0.81 for females, having a sedentary lifestyle (exercising two times or less per week).
The participants should fail to meet the healthy parameters of visceral adipose tissue, identified as 762 cm3 for males and 256 cm3 for females, evaluated by DXA scans. Additional exclusion criteria were also taken into consideration.
Recruitment of the participants was conducted through Atlantia’s database, the offices of General Practitioners, social media platforms and advertisements placed in local media.
Participants meeting the eligibility criteria were randomized (ratio 1:1) to one of the two treatment groups. Consent was informed in prior writing: capsules contained either the optimal dose (as per the sponsor's pre-clinical results) or a placebo.
Each participant deemed eligible was allocated a randomization number in chronological order, which represented one of the two groups.
The relationship between the group assignment and the randomization number was unknown to the clinical research team, study site staff, the participants and the sponsor.
The study required five visits per participant over a 13-15 week period, including a run-in phase and requisite intervention period. Participants were advised to consume one capsule before breakfast each day for the length of the study; if compliance was not observed, they were subsequently retrained.
During certain visits, a fasting blood sample (21 mls) was taken. For future exploratory evaluation of inflammatory and satiety biomarkers, additional plasma and serum were stored at temperatures of -80oC at Atlantia’s facilities.
Participants were provided with instructions for collecting and storing stool samples at home, as well as a stool collection kit. When participants returned the stool sample, they were stored at -80oC for future exploratory evaluation, including SCFA analysis, shotgun sequencing and detection of the strain.
Future plans and return on investment
Review of the trial data is ongoing and details on the results will be presented in the near future. The sponsor was extremely satisfied with Atlantia’s management of the trial, from development to performance.
About Atlantia Clinical Trials
Atlantia Clinical Trials Ltd is a CRO that specializes in conducting studies in foods, beverages and supplements for companies world-wide that want to scientifically validate their functional ingredients to support an: EFSA (European Food Safety Authority) Health Claim; FDA (Food & Drug Administration) Structure Function Claim; or General Product Marketing Claim.
Atlantia works with world leading scientists (among the top cited 1% internationally, in the areas of digestive health and functional foods) at the: APC Microbiome Institute in University College Cork, Ireland; Teagasc, Moorepark, Ireland and recognized centers of excellence globally.
Atlantia runs and operates its own clinic sites and conducts all studies to ICH-GCP standard (International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use - Good Clinical Practice).
Its team includes physician experts in digestive health, mental health (psychological stress and cognition), cardiovascular health, sports performance, metabolic disease, bone health, immune health and healthy ageing.
The clinical team also includes project managers, research nurses, nutritionists, certified sports trainers and lab researchers.
Atlantia manages all elements from protocol design, placebo manufacture, recruitment, and study execution, to sample and data analysis, statistics and report/dossier preparation to provide a service which is technically, scientifically and clinically superior.
The clinical studies cover a broad spectrum of functional food and beverage categories, such as dairy, cereal, probiotic, different protein forms, infant-specific foods, vitamins/minerals, plant or marine extracts and medical foods.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.