The body’s first response to SARS-CoV-2 is completed by the innate immune system and, soon after, the adaptive immune system. Innate immune cells recognize and remove the viral material while simultaneously signaling to other cells.
This signaling subsequently arouses host immunity to prevent viral spread from the infected cell to others nearby. The innate immune response often appears sufficient to eliminate the virus altogether, particularly in mild or asymptomatic infection.
Following this event, T-cells are recruited to destroy infected cells. This can only be achieved when the T-cells recognize the antigens presented by major histocompatibility class (MHC) class I and II molecules.
At the same time, the antibody immunoglobulin M (IgM) response develops. IgM is a multivalent antibody that is key to immune control in early infection. Memory B-cells are also formed at this time, both unswitched IgM+ and classical switched cells, and can persist for months.
Memory T-cells are also found in convalescents and react to many different SARS-CoV-2 epitopes. Interestingly, these cells are also found in SARS-CoV-2-naïve individuals, thereby suggesting cross-reactivity between this virus and the human endemic seasonal coronaviruses. IgM+ B-cells with cross-reactivity are also found in some people, whereas the IgG antibodies are typically specific to the SARS-CoV-2.
IgG and IgA titers rise following the class-switching of B cells after the early IgM response. IgG antibodies remain stable for months or years. IgG titers often decline at rapid or slow rates depending on how high they rose, the subtypes, and the involvement of short- and long-lived plasma cells in the immune response.
Upon reinfection, memory B-cells produce a rapid immunologic response. However, this response can be reduced with mutations at the viral spike (S) antigen, which have been shown to impair antigen recognition. The extent of protection offered against other variants following exposure to one strain, and the various ways in which T- and B-cells act together to regulate this response, remains to be determined.
Antibody response
Several studies have reported that over 90% of people with COVID-19 develop IgG antibodies to the S antigen, even with mild infections. The antibody titers, however, vary widely, with some non-seroconverters that have been identified.
The time to seroconversion also varies widely, with a mean of 10 days. This also accounts for the period required for class-switching to occur, and for the antibody titers to rise above the limit of detection of the test.
Affinity maturation of SARS-CoV-2-specific B-cells has been shown to occur similar to that seen in other viral infections. Total and neutralizing antibody titers are highest with more severe disease. This is likely due to the higher viral load or prolonged viral replication, which sustains inflammatory-immune signaling that ultimately results in higher antibody production.
More severe COVID-19 cases also develop higher titers of mucosal-protective IgA antibodies. These may remain detectable for months after the viral tests become negative.
Age is regarded as a risk factor for severe COVID-19, and antibody production appears to be weaker in the elderly relative to younger people. Comparably, children appear to have more cross-reactive antibodies and a higher number of SARS-CoV-2-specific B-cells. Pediatric studies are important in defining the biology and transmission of SARS-CoV-2 and identifying safe approaches to education during this pandemic.
Not only are some anti-S antibodies cross-reactive to SARS-CoV-2, but the latter may also boost the levels of some antibodies elicited by the seasonal coronavirus infections. This suggests that various SARS-CoV-2 epitopes on the S antigen can stimulate memory B-cells generated by related viruses, though the clinical significance of this has yet to be determined.
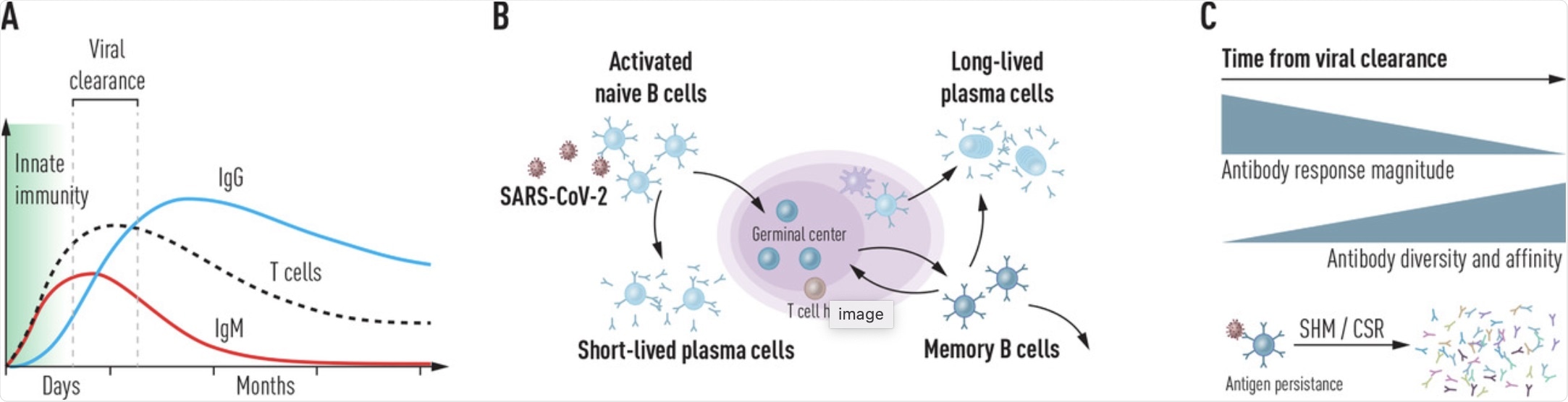 (a) Kinetics of innate and adaptive immune responses following SARS-CoV-2 infection. Following rapid action by the innate immune system immediately after virus exposure, B and T cell responses develop within the first weeks. Short-lived IgM antibodies are produced by responding B cells initially and is followed by a more persistent and high-affinity class-switched response. (b) Production of highly diverse virus-specific antibodies. After cognate antigen encounter, activated naïve B cells enter the germinal center (GC) where they receive T cell help to generate a high-affinity antibody repertoire. Short-lived peripheral plasma cells produce most antibodies during the infection, while GC-derived memory B cells and bone marrow-resident plasma cells cooperate to provide long-lasting protection against re-infection. (c) Maturation of the antibody response following viral clearance. While the magnitude of the antibody response gradually wanes after the virus replication is controlled, the quality of the B cell response continues to improve for several months following the infection.
(a) Kinetics of innate and adaptive immune responses following SARS-CoV-2 infection. Following rapid action by the innate immune system immediately after virus exposure, B and T cell responses develop within the first weeks. Short-lived IgM antibodies are produced by responding B cells initially and is followed by a more persistent and high-affinity class-switched response. (b) Production of highly diverse virus-specific antibodies. After cognate antigen encounter, activated naïve B cells enter the germinal center (GC) where they receive T cell help to generate a high-affinity antibody repertoire. Short-lived peripheral plasma cells produce most antibodies during the infection, while GC-derived memory B cells and bone marrow-resident plasma cells cooperate to provide long-lasting protection against re-infection. (c) Maturation of the antibody response following viral clearance. While the magnitude of the antibody response gradually wanes after the virus replication is controlled, the quality of the B cell response continues to improve for several months following the infection.
Antibody specificity
The SARS-CoV-2 S antigen produces a range of antibodies derived from multiple immunoglobulin heavy chain variable (IGHV) genes, many of which produce potent neutralization. In the early immune response, only a few V genes are used; however, the responses widen as the disease progresses and more B-cells are activated.
Some studies have shown common immunoglobulin germline V, D, and J gene usage by S-targeting neutralizing antibodies. These observations indicate that these are the preferred configurations that already have an adequate binding affinity.
Most neutralizing antibodies target the receptor-binding domain (RBD) of the S antigen, while some bind to the spike N-terminal domain (NTD). Others in the S2 domain of the S protein may be invaluable, as they have cross-reactive potential, while not being affected by RBD/NTD mutations.
Most studies show that the trimeric S protein elicits a range of antibodies derived from many IGHV genes that are often commonly engaged in other viral infections as well. These antibodies, which make up the general IgG repertoire, include IGHV1-69, IGHV3-23, IGHV3-30, and IGHV3-30-3.
Some common databases have been set up for antibody sequences to be deposited and searched to further research into humoral immunity in COVID-19 These include the Coronavirus-binding Antibody Sequences and Structures Database (http://opig.stats.ox.ac.uk/webapps/covabdab/) and The Coronavirus Immunotherapy Consortium (https://covic.lji.org). The goal of broadening the availability of this information is to support the timely development of therapeutic antibodies for COVID-19 treatment.
Immunosuppression, whether inborn or due to immunosuppressive therapy, may interfere with viral clearance and impair antibody production.
Immunity following natural infection
Immunity after natural infection is assessed in terms of protection against symptomatic and severe disease upon reinfection, rather than prevention of viral transmission. Some high-quality studies show that the rate of reinfection in the six months after natural infection was much lower than that which occurred in naïve people.
Immunity following vaccination
Vaccination with almost all vaccines that are currently in clinical use produce high titers of neutralizing antibodies that are correlated with protection against the virus. With high titers, neutralization persists, even in the presence of emerging variants that have higher resistance to these antibodies.
Many different types of vaccines have been approved, the most efficacious of which includes the messenger ribonucleic (mRNA) types. These vaccines, which have been developed by Moderna and Pfizer/BioNTech, encode the viral S protein after entering the host cell.
Other COVID-19 vaccines that are currently approved for use in many countries around the world include adenovirus vector vaccines, which introduce the SARS-CoV-2 S antigen and inactivated whole virus vaccines. Protein subunit vaccines are expected to be approved in the near future.
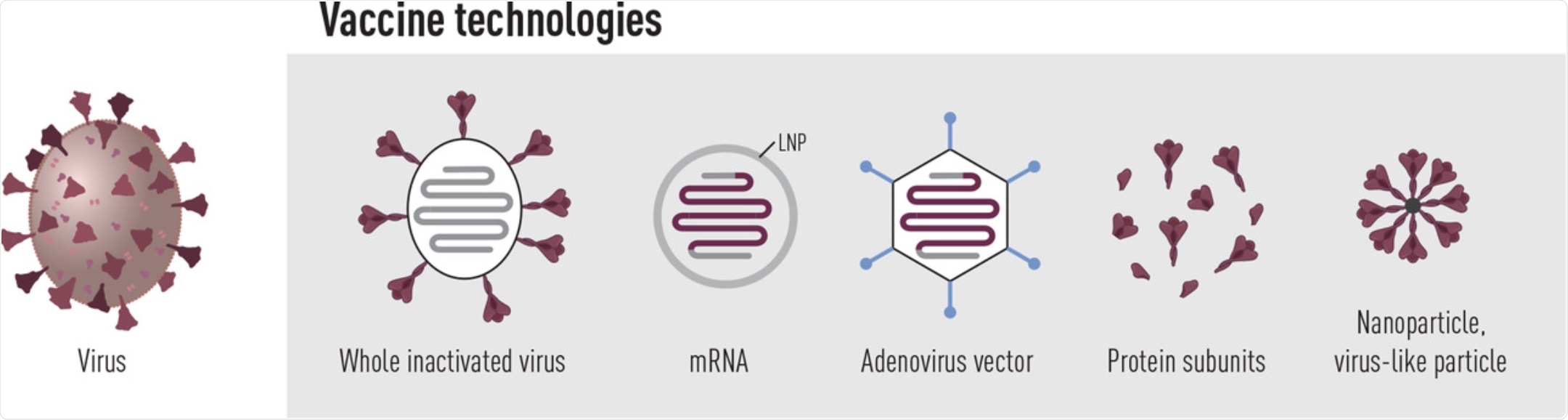 Vaccine platforms in use or under clinical evaluation to prevent COVID-19. Currently, several inactivated whole virus vaccines as well as SARS-CoV-2 spike-encoding vaccines based on mRNA or adenovirus technology are approved for clinical use. Furthermore, spike protein subunit vaccines have shown high efficacy in phase 3 clinical trials and should be approved for use shortly.
Vaccine platforms in use or under clinical evaluation to prevent COVID-19. Currently, several inactivated whole virus vaccines as well as SARS-CoV-2 spike-encoding vaccines based on mRNA or adenovirus technology are approved for clinical use. Furthermore, spike protein subunit vaccines have shown high efficacy in phase 3 clinical trials and should be approved for use shortly.
All approved vaccines have shown the ability to induce neutralizing antibody titers comparable to those which are elicited by natural infection, or higher. These vaccines provide very high levels of protection against moderate to severe COVID-19.
In real-world data, Pfizer vaccination in Israel reduced symptomatic infection by up to 74% with one dose, and up to 95% with two. In the United Kingdom, corresponding figures with the same vaccine were 70% and 85%. With respect to the SARS-CoV-2 Delta variant, data from the U.K. claims 75% efficacy in preventing hospitalizations due to infection.
Notably, Israeli data seems to indicate that mass vaccination protects children under 16 as well, especially after two doses.
Further analysis of vaccine-related adverse responses is essential to dispel public doubts and extend the reach of vaccination where required. These studies will also provide a better understanding of how vaccine efficacy declines over time.
Immunity against variants of concern
SARS-CoV-2 variants of concern (VoCs) have been emerging since the onset of the pandemic.
Though all current vaccines, which were developed based on the Wuhan strain of SARS-CoV-2, show a loss of efficacy in preventing symptomatic disease with the Delta variant, the benefit is still pronounced. However, the waning of antibody levels with time makes virus monitoring a top priority, with the potential need for booster doses, especially in the elderly.
This is being considered in Israel, before the winter season. Nonetheless, the researchers note, there are two promising features:
1) “Even if some classes of [antibodies] Abs lose reactivity, the overall anti-S Ab response, including to the RBD, is polyclonal and consists of Abs that also recognize the VoCs.”
2) T-cells that target this virus are relatively unaffected by the VoC mutations, though these are not able to provide protection against reinfection, as typified by the need for annual updated flu shots.
3) The Beta VoC appears to provide protection against reinfection with the Wuhan strain and the Alpha (B.1.1.7) lineage. This may indicate that the S protein mutation in this strain is promising for second-generation vaccines, especially with newer platforms.
Some governments are exploring the utility of a heterologous vaccine protocol, as this may broaden the reach of immunity. Two doses appear to be required to achieve high antibody titers and better cross-neutralizing activity against VoCs. Careful monitoring will be required to determine how and where new variants arise and the ability to neutralize them by vaccine-induced antibodies.
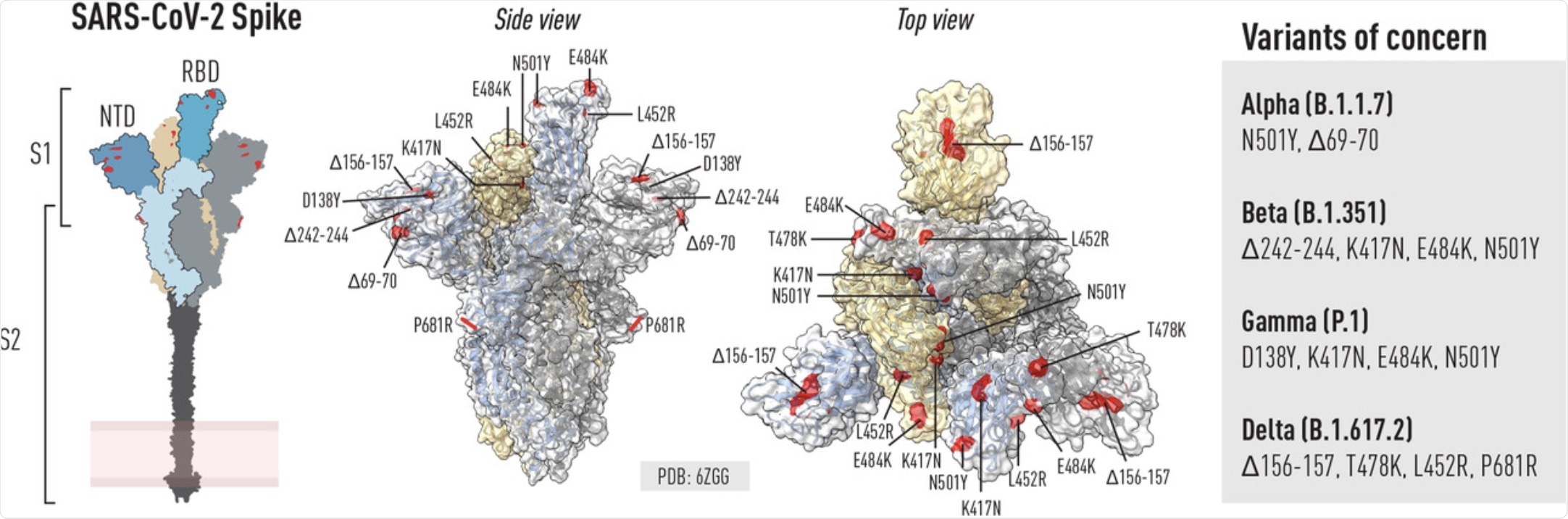 Schematic of the SARS-COV-2 spike glycoprotein and location of mutations in VoCs. Left: Schematic of the spike trimer highlighting key domains including the RBD, NTD and S2. Right (side and top view): ChimeraX was used to illustrate selected residues in the spike trimer that are mutated in selected VOCs known at the time of writing. Mutations can be common or unique to different VOCs.
Schematic of the SARS-COV-2 spike glycoprotein and location of mutations in VoCs. Left: Schematic of the spike trimer highlighting key domains including the RBD, NTD and S2. Right (side and top view): ChimeraX was used to illustrate selected residues in the spike trimer that are mutated in selected VOCs known at the time of writing. Mutations can be common or unique to different VOCs.
Vaccination post-COVID-19
“Protective immunity after SARS-CoV-2 infection is generally good, and the risk to be re-infected is low, although not non-existent.”
The presence of comorbidities, age, and the presence of VoCs affect the risk and, even more importantly, the time since infection and the peak antibody titer attained.
Post-infection, a single vaccine dose can cause clonally expanded immune responses, with cross-neutralizing antibodies covering VoCs as well. Vaccine-induced antibodies mostly target the S antigen RBD, unlike those resulting from infection.
What are the implications?
The future of the pandemic is uncertain. It is possible that SARS-CoV-2 may become endemic and less pathogenic, more like the flu. Conversely, it may become more virulent and infective as a result of mutations.
“In the future, it is possible that annual vaccination against SARS-CoV-2, analogous to seasonal influenza vaccines, will be recommended for some age groups, depending on disease susceptibility and the magnitude of community transmission. Furthermore, it will be important to address in large future studies, what Ab titers are required for protection against disease or infection.”
Newer and improved antibody tests also need to be developed in order to enhance their accuracy while being suitable for mass testing. Such monitoring is crucial to truly evaluate vaccine efficacy and ensuring that people in various occupations are not susceptible to or transmitters of COVID-19, including those who care for the elderly in residential facilities.
“Despite the global challenges posed by SARS-CoV-2, the pandemic provides an unrivalled opportunity to learn about viruses and the human immune system at the population level and improve pandemic preparedness through global cooperation.”