Oct 20 2016
In a collaborative study, scientists from MIPT and JINR (Joint Institute for Nuclear Research) have increased the accuracy of detecting valuable protein crystals measuring just a few microns. These small crystals are now used to study the structure of membrane proteins. Knowledge of these proteins is very important for fundamental and applied pharmaceutical research. A paper on the study has been published in the prestigious Journal of American Chemical Society.
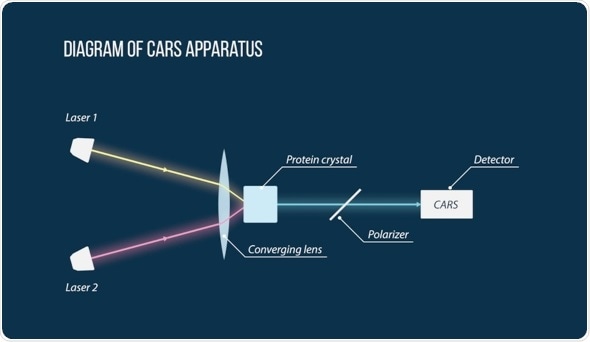
A diagram of the CARS apparatus used by the scientists to detect the small protein crystals. Two laser beams (Laser 1 and Laser 2) intersect on the sample. The signal received from the highlighted region passes through several filters (including a polarizer) and hits a detector. With further processing, scientists are able to say whether there is a protein crystal in the region or not. Image courtesy of MIPT Press Office.
Proteins: molecular machines useful for pharmacists
Proteins are important molecular machines that perform most of the functions in the human body. Of particular importance are membrane proteins, which “sit” on the membrane of every cell in our bodies—they carry substances, energy, and signals, directly into a cell, enabling it to “communicate.” Membrane receptor proteins are the most popular targets for drugs. According to various estimates, up to 60% of all modern drugs target these proteins—e.g., Nasivin or Plavix (a drug used to reduce the risk of strokes). By activating or deactivating receptors, the correct drugs can regulate physiological processes that have been affected during an illness.
Knowing the structure of a protein makes it much faster and cheaper to find a drug. A detailed structure can be obtained by X-ray diffraction; a large homogeneous protein crystal needs to be grown for this. The scattering of X-rays on the crystal lattice then gives the structure of the protein. Obtaining a crystal of a membrane protein is very difficult—traditional methods of crystallization used to produce crystals of water-soluble proteins do not give the desired results. It is especially difficult for GPCR receptor proteins, which are very important—in 2012, a Nobel Prize was awarded for a study of their structure.
To study membrane proteins, scientists currently use X-ray free electron lasers—highly advanced sources of hard X-rays. They are powerful enough to be used on very small crystals. The problem is that when growing crystals of this size it is difficult to know if they are good quality or if they have grown at all. To pretest the quality, scientists currently use the SONICC technique, which is based on imaging from the SHG method (which allows scientists to “see” ordered crystals through a disordered medium in which they are growing) and UV-TPEF (a special type of microscopy that can only see special amino acids present in protein molecules). However, the accuracy of this method is often insufficient to detect crystals that are about the size of one micron. Scientists from MIPT and JINR have succeeded in achieving better results than the SONICC method (or rather its main component, SHG), while also demonstrating sensitivity to the subtle features of a protein structure that will be extremely important in further research studies.
CARS: detecting micron crystals with greater accuracy
To study the crystals, scientists used the P-CARS method—a special kind of spectroscopy. It is based on the nonlinear optical effect and can notably be used for high resolution imaging of processes occurring within cells. The method requires two lasers, the beams of which are crossed on a sample with a protein crystal. The resulting signal is filtered by an optical system, which enables scientists to distinguish the regions with the protein crystal from the non-protein environment. By scanning individual points of the sample one at a time, researchers obtain a three-dimensional image of the crystal.
CARS microscopy, which is mainly used to visualize processes taking place in cells, is widely known in the scientific community. The CARS method can also be configured to detect chemical bonds that are specific only to proteins, allowing scientists to see “through” a medium which crystals are growing in. In their study, the researchers applied the method to the model proteins bacteriorhodopsin (a typical membrane protein) and lysozyme (a typical water-soluble protein).
It was found that with bacteriorhodopsin crystals a common crystal defect known as twinning can be detected early on. This is not possible when using SHG—the main component of the SONICC method (which “sees” ordered crystals without distinguishing their structure). Twinning often means that a protein structure cannot be identified with sufficient accuracy, making it more difficult to find new drugs. Detecting this defect in microscopic crystals was, until now, only possible by conducting costly X-ray studies. With the CARS method, the defect can be found quickly and easily.
Using lysozyme crystals as an example, the scientists demonstrated a significant advantage of the CARS method by showing that in certain cases it can see crystals that are not visible using the SONICC method.
Prospects: new-generation equipment
The researchers applied the recognized CARS method to detect and study protein crystals and showed that in terms of accuracy and sensitivity to important crystal defects it is superior to the SONICC method which is used currently. Given that CARS is no more expensive or more complex than SONICC, it can be expected that it will soon be used in the next generation of commercial crystallization equipment. In addition, the fundamental results obtained will improve the quality of the crystallization of membrane proteins, which are important for pharmacology, by making it easier to develop and produce new drugs that have minimal side effects and are more effective.