Researchers are reporting the impressive results of a new cell and gene therapy that has achieved unprecedented success in treating multiple myeloma patients.
Although the study is an early one and the population only included 35 people, every one of the patients responded to the treatment and 33 (94%) were in some degree of clinical remission within two months.
T-cells attacking cancer cell illustration of microscopic photos.
The findings were presented yesterday at the American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, where experts were describing the results as a first for multiple myeloma and a rarity for cancer treatment in general.
“It’s rare to see such high response rates, especially for a hard-to-treat cancer,” said ASCO Expert Michael Sabel. “This serves as proof that immunotherapy and precision medicine research pays off. We hope that future research builds on this success in multiple myeloma and other cancers.”
The therapy involves chimeric antigen receptor (CAR) T cells targeting B-cell maturation protein (BCMA), a protein that was found to play a role in multiple myeloma progression back in 2004.
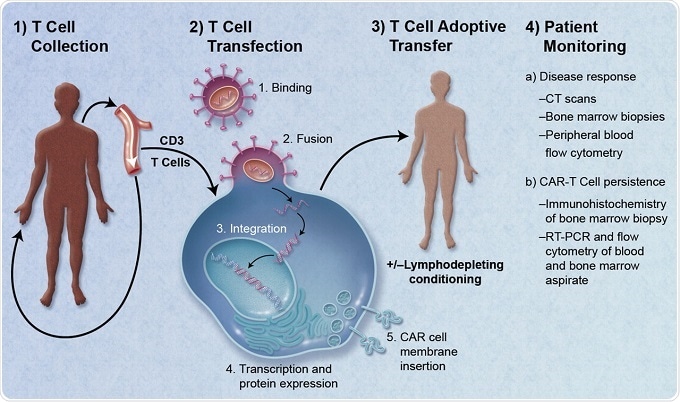
Depiction of adoptive cell therapy using CAR-modified T Cells.
Credit to: Caron A. Jacobson and Jerome Ritz
The therapy is individualized to each patient. Their own T-cells are collected and then altered to contain a gene that targets cancer. The permanently altered cells are then injected back into the patient’s body, where they multiply to create an army that can find and destroy cancer cells.
The current findings are the results of an ongoing phase I clinical trial in China in which 35 patients with relapsed or treatment-resistant multiple myeloma have been administered the therapy.
Overall, 100% of patients responded to the treatment and 33 had either a complete response or a very good partial response within two months.
To date, 19 of the patients have been followed long enough for full efficacy of the treatment to be assessed (more than four months, as set by the International Myeloma working Group) and 14 have met the criteria for complete remission. Four of the patients met the criteria for very good partial remission and one met the criteria for a partial response.
The majority of the patients experienced side effects that are common with the therapy including fever, difficulty breathing and low blood pressure, but only two cases were severe and all were treatable and short-lived.
According to Sabel, the treatment is "revolutionary", also commenting "This is really the epitome of personalized medicine."
Next, the researchers plan to include 100 patients in the trial from four hospitals in China and to launch a similar trial in the U.S. in 2018.
One of the authors of the study, Wanhong Zhao (Xi’an Jiaotong University, China), said: “Looking ahead, we would also like to explore whether BCMA CAR T-cell therapy benefits patients who are newly diagnosed with multiple myeloma.”
This study was funded by Legend Biotech Co.