An interview with Prof. David Alsteens, Université Catholique de Louvain conducted by April Cashin-Garbutt, MA (Cantab).
Can you please give a brief introduction to your research using AFM to image single proteins, receptors, viruses and cells?
We study mammalian cells and try to understand how these cells interact with their environment. We mainly focus on the interactions that can occur with individual ligands.
We look at how single ligands can interact with a receptor and trigger an intracellular signal, or interact with viruses to see how the virus binds to our cells, and then try to internalize and indulge the cells to divert a genetic cargo.
Measuring Cell Interactions with AFM
Measuring Cell Interactions with AFM from AZoNetwork on Vimeo.
How do you use force-distance curves based-AFM to probe molecular or cellular biophysical properties to quantify at the single-molecule level interactions that drive biological processes?
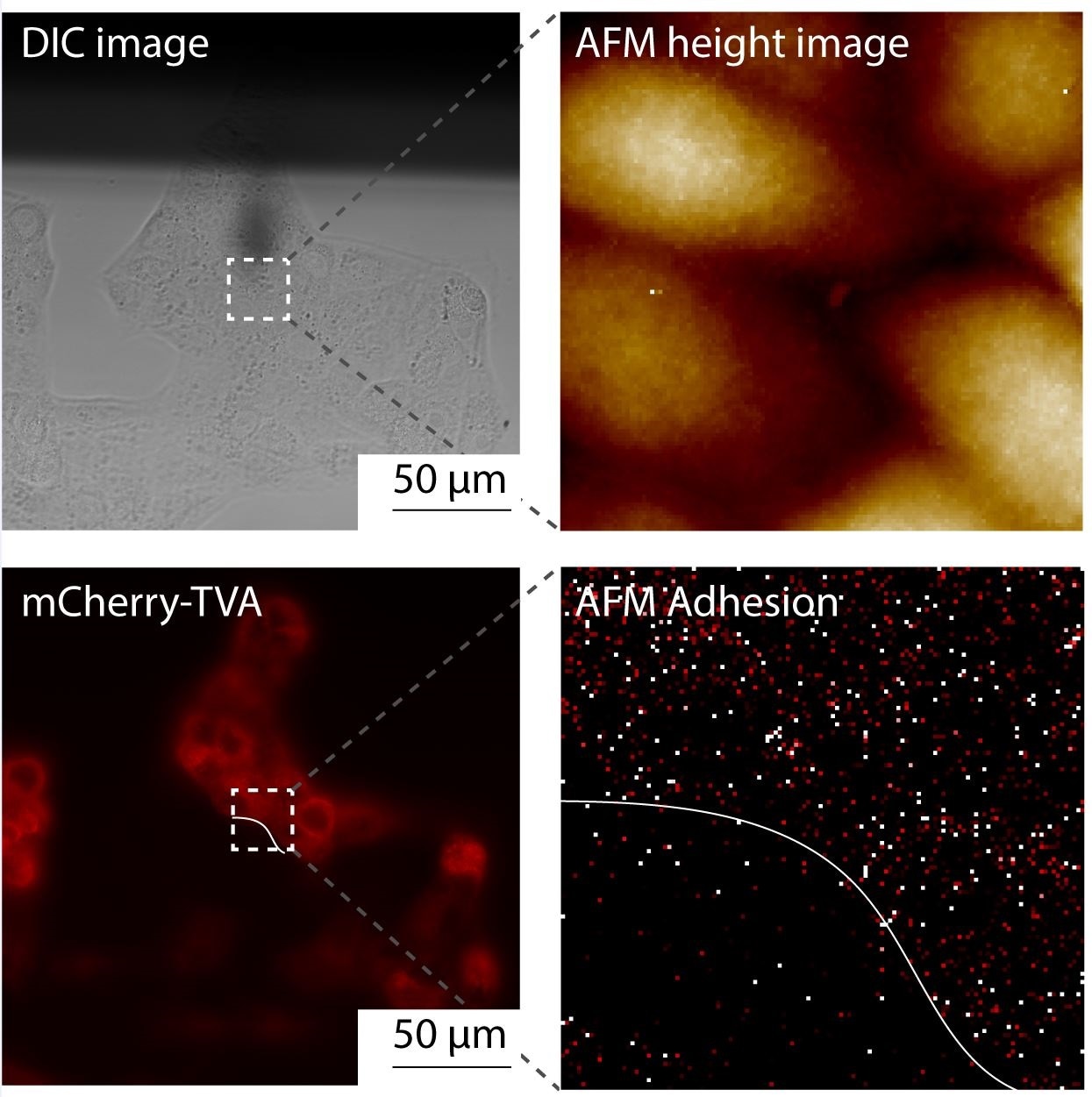
The idea is to functionalize the tip by grafting some molecules on the AFM tip apex. You can theter a single ligand or theter a single virus onto the tip. You then put this tip in contact with the surface to measure the interaction between the ligands and the cell surface receptors or the interaction between a single virus and the host cell surface.
The difficulty with the cells is that it's mandatory to control their environment − the temperature, the humidity and how the cells are moving. The conditions always need to be good so that the cells stay alive and you are able to probe the interaction and be sure that the cells behave as they would in a physiologic environment.
Another issue is that cells are quite soft, so it is very difficult because you want to just touch the surface, but without destroying the cells, which are very fragile.
How has studying biological processes in the cellular context extended and changed our understanding of cell surface biology?
Quite recently, we have been able to probe single interaction on cells, so that we have a better idea about how a single ligand is able to interact in vivo. Our measurement are performed on isolated cells, however we can assume that a similar kind of interactions occur within the organism during fundamental biological processes.
As en example, we are able to see, during the first instant of the interaction of a single virus with a cell surface, how many bonds are established and what the dynamic of these bonds is.
For the first time, we were able to probe specific interactions directly on living cells. This provides us with details about the number of interactions established during the first contact between a virus and a cell, for example. It tells us the importance of bond establishment, their valency, their affinity.. all the factors mandatory to trigger an effective infection mechanism.
How has AFM directly advanced or helped your research?
Since my PhD, I have always tried to push the limits of AFM with the idea to measure single-molecule interaction on living cells. I started this technique to microbiology and now I am studying animal cells.
Thanks to our recent developments we are able to address key biological question at the cellular level and understand how individual ligand or virus mediate cellular response. Recently, new force-distance curve based-AFM has enable faster measurement and allows the applied force to be more precisely controlled.
What is the biggest impact that AFM has made to the biological and nanomedicine research fields?
I think with the recent development, we can now study new mechanisms that occur on living cells. By understanding how, a virus interacts with cells, for example, we will see which cellular components is very crucial during the first steps of infection.
Then, in a second step, we will be able to develop and target this first interaction key events to abolish these preliminary interactions. I think that quite soon in the future, we will be able to develop some drugs that target this first interaction.
How has Bruker technology helped or advanced AFM in biological research?
I would say that this recent technological development has enabled us to image living mammalian cells, which was not feasible with the previous modes. At the same time, it enables us to measure specific binding events on cells, meaning we can directly correlate the topography with the binding site and extract the kinetics and the thermodynamics of the interaction, therefore providing a full picture of the interaction that take place on cells.
Which system do you use?
Currently, we are working in three different fields. We are working in virology and studying the interaction between a virus and cells. We are looking at specific receptors and seeing how, for example, a GPCR interacts with a single ligand to activate an intracellular signal.
We are also interested in the organization of lipids on red blood cells or other cells and looking at whether the lipids are homogenous or heterogeneous distributed and if they assemble in nanodomain like rafts or are more stable on cells.
What is the importance of meetings, like the AFM Biomed Conference, to you and the AFM research community?
I think it is nice to share our research results and discuss them with other people in order to push ideas that you cannot expand in your own lab. It is a way to meet people and to look at the new developments.
What direction do you see, or would like to see, AFM going in the next five years? What do you see as the next big thing for AFM?
I think it's now time to apply the developed technology so far to address unexplored biological questions. Studying most of biological mechanism related to surface interactions does not need to develop new modes; we can already t use what is currently availbale to answer very big questions, which would help directly in the targeting or screening of drugs and anti-adhesive molecules.
I have been in my institute for two years now and I discuss with my colleagues what the most powerful or important part of AFM is and how I can help them. I recently observed that many biologists from different fields start to see the unique capabilities of AFM and how they can use them to circumvent current limits in their research.
I recently worked with a colleague in immunology who had a receptor on cells that he was not so sure of in terms of how a ligand interact with. He knew that signalling was mediated by this specific receptors but not sure whether this receptor was directly interacting with the ligand. This is the typical question that we can address with our technology.
To study it, we grafted the ligand on the AFM tip and we measured the interaction. We were able to measure a specific interaction and even more, that specific interaction was triggering an intracellular signal and a cellular response.
We can already apply this technology to other fields and help biologists, for example, or people in medicine to answer big questions.
Do you think AFM will have clinical applications moving forward?
I think it is quite difficult to implement AFM within a hospital because it is quite a challenging technique. From a scientist’s point of view, it is quite difficult to use the current AFM technology. A new researcher in my lab will take about six to nine months or even a whole year to understand all the parameters of this technology.
However, I think it is quite a useful technology because there are a lot of parameters that you can extract. You can image the topography; you can get information about a specific interaction; you can extract nano mechanical properties and modular stiffness.
Combining all this data can provide a full picture of what occurs on living cells and answer a lot of questions. However, training people who have never used the technology to use these tools is quite challenging!
Where can readers find more information?
About Prof. David Alsteens

Prof. Dr. David Alsteens is now a head of a NanoBioPhysics group at Université catholique de Louvain in Belgium. David has acquired his MSc. and PhD at the same University in the field of nanobiothechnology with Prof. Yves Dufrêne. After that David did a PostDoc at Department of Biosystems Science and Engineering at ETH in Basel, Switzerland in the group of Prof. Daniel Muller.
David’s research focus at the moment on the NanoBioPhysics of the cell surface machinery. His team uses mainly use atomic force microscopy (AFM) to image at high-resolution single proteins, receptors, virus and cells. Furthermore, he uses force-distance curves based-AFM to probe molecular or cellular biophysical properties to quantify at the single-molecule level interactions that drive biological processes.
He just received an ERC Starting grant from the European Research Council to study virus infection mechanism using AFM and confocal microscopy.
David is the co-author of 60 publications and 7 book chapters.