A preclinical study with mice has shown that Gabapentin, a drug that is commonly used in nerve pain could be useful for patients with spinal cord injury. Use of the drug showed improvement in upper limb function among the mice models of spinal cord injury, wrote the researchers. The study titled, “Gabapentinoid treatment promotes corticospinal plasticity and regeneration following murine spinal cord injury,” was published this week in the latest issue of the Journal of Clinical Investigation.
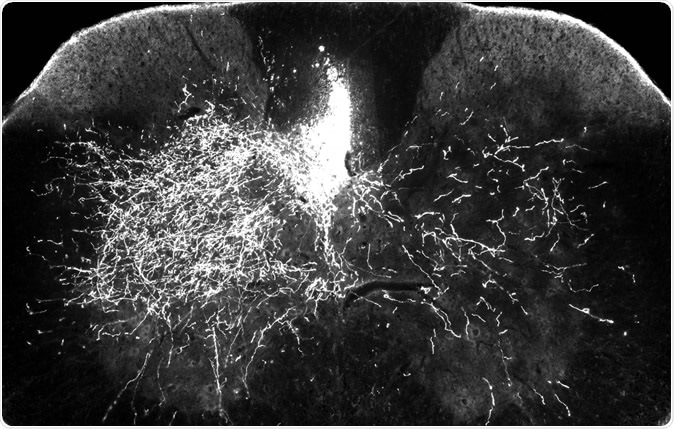
In one of the mouse models for this research, one side of the descending spinal pathway is damaged (above right) while the other side (above left) remains intact. In this image, bright streaks on the right show that in mice treated with gabapentin, descending motor axons are able to sprout to send nerve impulses to the denervated part of the spinal cord – a key step in rebuilding the central nervous system after injury.
For the study the team included mice with spinal cord injury. The mice that had been treated with gabapentin showed around 60 percent improvement in the forelimb functions as seen in a skilled walking test. Mice that served as control and were given a placebo showed a 30 percent improvement in the test said the researchers. They focussed on the nerves that controlled the voluntary movements of the muscles of the body. These nerves mainly are present at the corticospinal tract, they wrote.
Lead author Andrea Tedeschi, assistant professor of neuroscience at The Ohio State University said, “There is some spontaneous recovery in untreated mice, but it’s never complete. The treated mice still have deficits, but they are significantly better. This research has translational implications because the drug is clinically approved and already prescribed to patients. I think there’s enough evidence here to reconsider how we use this drug in the clinic. The implication of our finding may also impact other neurological conditions such as brain injury and stroke.”
The team explained that gabapentin could block a specific protein called alpha2delta2 within the nerves that stops the growth of the nerve cell bodies or axons and increases formation os synapses. After an injury normally there is an increased activity of the protein that increases pain and reduces axon growth. If this protein is blocked, the axons and thus the nerves are allowed to grow and this can help restore the nerve function of the limbs, they explained. Tedeschi explained, “When neuronal circuits need to be rebuilt after injury, we need to down-regulate the expression of the receptor so axons can re-engage in an active growth program. And we found that it’s doing exactly the opposite.” “Because this receptor can be pharmacologically blocked through administration of clinically approved drugs called gabapentinoids – for example, gabapentin and pregabalin – that’s a very powerful target that you can modulate as long as you take the drug,” he added.
Wenjing Sun, research assistant professor of neuroscience at Ohio State, who was also the first author of the study also said, “We really have to consider that rebuilding neuronal circuits, especially in an adult central nervous system, takes time. But it can happen.”
The authors of the study explain that the injury they created in the mice was at the top of the spine and in humans with similar injuries, there is complete debilitation and these individuals would require continued assistance with their day to day activities. The mice were similarly unable to move after the injury. They were administered gabapentin for four months. Those on treatment could, at the end of the study, move across a horizontal ladder and spread their forelimbs and toes.
To confirm that their drug was indeed stopping the protein and allowing the nerves to grow, the team next stopped the repair pathways of the neurons. Thereafter no differences were found between gabapentin treated and untreated mice. Tedeschi said, “Now we can comfortably say that whatever we see in terms of structural and functional alterations of this motor pathway is really meaningful in promoting recovery in these mice,”
The authors wrote in conclusion, “Mice administered gabapentin recovered upper extremity function after cervical SCI. Importantly; such recovery relies on reorganization of the corticospinal pathway, as chemogenetic silencing of injured corticospinal neurons transiently abrogated recovery. Thus, targeting α2δ2 with a clinically relevant treatment strategy aids repair of motor circuits after SCI.”
“Gabapentin is given when the nervous system is already having issues associated with maladaptive plasticity that hinders normal function. We are giving it much, much earlier, when the nervous system may be more responsive to programming an adaptive repair process,” he said. This meant that for humans with spinal injury as well, gabapentin needs to be started early for benefits to be proven.
The team is planning to translate their findings into human clinical trials next. Tedeschi said, “With all the evidence and mechanistic insight we provide, I feel like we are in a better situation to start planning a more translational type of research. It’s the right time to try.”
The study was funded by the Craig H. Neilsen Foundation, Marina Romoli Onlus Association, Ohio State University Neuroscience Research Institute, National Institute of Neurological Disorders and the National Institutes of Health.
Journal reference:
Gabapentinoid treatment promotes corticospinal plasticity and regeneration following murine spinal cord injury, Wenjing Sun, Molly J.E. Larson, Conrad M. Kiyoshi, Alexander J. Annett, William A. Stalker, Juan Peng, Andrea Tedeschi Published December 3, 2019, J Clin Invest. 2019. https://doi.org/10.1172/JCI130391, https://www.jci.org/articles/view/130391