In this interview, Jason Lin Ph.D. Application Scientist from Wyatt Technology, talks to News-Medical about the new ultraDAWN that is a breakthrough in monitoring nanoparticle biopharmaceutical and polymer product process.
Please tell us about the ultraDAWN for measuring molar mass and size in real-time?
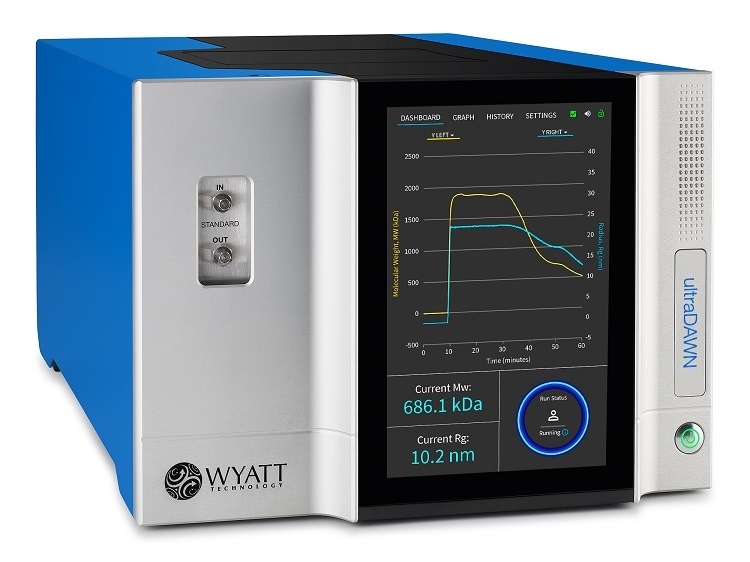
UltraDAWN is a multi-angle light scattering (MALS) detector for real-time monitoring of weight-average molar mass (Mw) and radius of gyration (Rg). It integrates into the production environment, serving as a process analytical tool for biopharmaceuticals, polymers or nanoparticles.
In addition, the OBSERVER software that accompanies ultraDAWN can be configured to trigger automated process control. A trigger might be set up to halt a reaction when the endpoint has been reached, or to divert a product stream to waste when a CQA deviates from its allowable range. Real-time MALS (RT-MALS) with the ultraDAWN is a means to maximize product yield and quality.
How is the ultraDAWN a breakthrough in process analytical technology for the production of nanoparticles, biopharmaceutical and polymers?
Typical process analytical technologies monitor process variables such as temperature, pH, pressure, or feedstock depletion. However, ultraDAWN measures product attributes—relevant properties of the product, rather than the process—that are directly related to quality and CQAs.
The UltraDAWN is a breakthrough process in analytical technology for the production of nanoparticles Image Credit:Shutterstock/SebastianKaulitzki
Was there a gap in the market for this product? What sets it apart from other models on the market?
There definitely was a gap in the market, as evidenced by the intense interest exhibited by scientists and engineers involved in manufacturing relevant products. Currently, there are no other PAT instruments that can directly quantify these CQAs in real-time. UltraDAWN holds the potential to save a lot of time during process development and to save substantial amounts of money by maximizing yield and flagging CQA drift before product leaves the manufacturing floor.
Why is the ultraDAWN the solution for particulate contamination and GMP manufacturing? What are the main features and benefits?
The ability to detect particulate contamination is a side benefit of RT-MALS, which results from the high sensitivity of light scattering to the presence of a small number of large particles. Other on-line detectors, based on UV/Vis absorption, refractive index measurement, fluorescence, Raman scattering, etc, do not offer this enhanced sensitivity and would usually miss such contaminants. Whether monitoring molecular weight or size, contamination by just a few large particulates will cause an appreciable jump in the signal.
In the current ultraDAWN model, GMP manufacturing is supported by the on-line RT-MALS configuration, which takes a low-volume feed of product from the process bulk. After measurement, the feed goes to waste so as not to impact sterile operation. In addition, the OBSERVER software for RT-MALS communicates via OPC and can be controlled by an OPC-AU client for FDA 21CFR(11) compliance. Whether monitoring molecular weight or size, contamination by a just a few large particulates will cause an appreciable jump in the signal. Future models may provide additional features for GMP support, e.g. single-use flow paths for in-line operation.

How will the ultraDAWN help pilot labs scale-up to manufacturing?
Process development can be a laborious task, running through a matrix of process variables and shipping many samples to the analytical lab, then waiting for feedback. With ultraDAWN and RT-MALS, production engineers receive immediate feedback on tweaks made to process variables. Indications regarding the need for troubleshooting and optimization can be recognized on the spot. The instrument can then be left in place for spot-checking quality or for continuous monitoring and control.
Which applications will benefit from the new product? How?
The most immediate applications that have generated interest are de-polymerization of polysaccharides for vaccines and production of nano-drug delivery formulations. Continuous downstream processing of monoclonal antibodies is another important use of RT-MALS for PAT.
Image Credit:Shutterstock/ustas7777777
Can you tell us about any case studies where the ultraDAWN has been used?
Polysaccharides used in vaccines start out with a fairly high molecular weight, nearly 2 MDa, and must be reduced to about 350 kDa for better immunogenicity, delivery and stability profiles. De-polymerization is accomplished with a variety of methods, but the application of ultrasound is a popular one. In one case study, an HPLC pump was controlled by OBSERVER software to dilute and feed a continuous, low-volume stream of API to the ultraDAWN during ultrasonication. The weight-average molecular weight was monitored, and when it was solidly within the acceptable range, a trigger was automatically sent to halt the reaction. Rather than waiting a fixed amount of time and hoping that the process proceeded exactly as it did during scale-up, production staff could now be confident that the final material met molecular weight specifications, even before it was sent to the QC lab.
In another case study pertaining to liposome production, size was monitored in-line with a microfluidization chamber that produces homogeneous particles. When chamber pressure was maintained at the specified level, ultraDAWN measurements found the liposome size constant to within 2%, well within specifications. However, when the chamber pressure drifted, so did the size. In a simulated failure of the chamber’s pressure control, OBSERVER detected the excess size deviation and diverted the process flow exiting the microfluidization chamber to a waste container, so the incorrectly sized particles did not contaminate the particles that were in spec.
Image Credit:Shutterstock/luminancestudio
What does the new product mean for the future?
As the ultraDAWN is incorporated into more production lines, manufacturing staff will experience greater productivity. They will be able to develop new processes more rapidly. They will rely less on maintaining specific, tightly-controlled process variables (even if those do not really impact the final product quality) since they will have a direct window into product properties. They will see fewer rejects by the QC lab and will pump out more product, with higher quality, in less time.
Where can our readers go to find out more?
A great place to start is on the Wyatt web site, by visiting the RT-MALS page (www.wyatt.com/RT-MALS) and the ultraDAWN page (www.wyatt.com/ultraDAWN). For additional details, readers are welcome to e-mail us at [email protected].
About Jason Lin Ph.D.
In Jason’s role at Wyatt, he utilizes a variety of light scattering techniques to investigate samples for biopharmaceutical companies, chemical companies, academic centers, and government research labs. Jason obtained his Ph.D. from the University of California Santa Barbara's Department of Chemistry.
.jpg)