In this interview Dr Kushol Gupta, Research Assistant Professor in the Department of Biochemistry and Biophysics at the Perelman School of Medicine, University of Pennsylvania talks to News-Medical and Life Sciences about the importance of light scattering techniques for investigating structure and mechanism of action in his ongoing research into HIV integrase and the development of HIV therapeutics.
Which instrumentation do you consider key to your research?
In the John Foundation Core, we rely upon an array of state-of-the-art instrumentation. All the techniques we use come together and complement each other to provide a deeper understanding of structure and function.
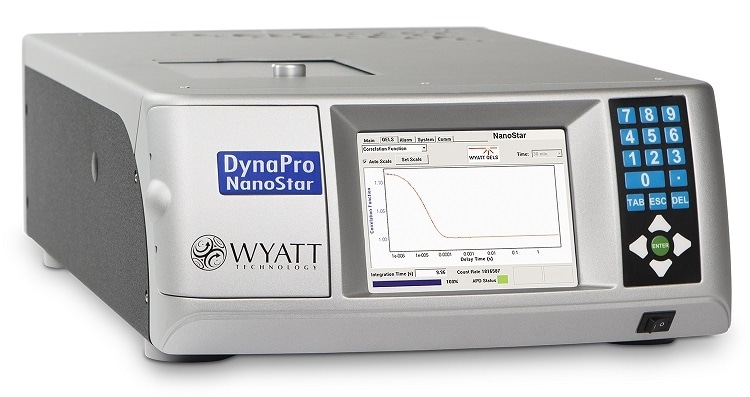
Light scattering measurements are key enablers of our ongoing research. In fact, the most popular and productive workhorses of our toolbox, day to day, are our light scattering instruments, all made by Wyatt Technology. These include our Dynapro Nanostar dynamic light scattering (DLS) instrument; our SEC-MALS components, the DAWN multi-angle light scattering instrument and the Optilab refractive index detector coupled to size-exclusion chromatography; and our most recent acquisition, our CG-MALS (composition-gradient multi-angle light scattering) instrument, the Calypso.
Over the past decade, the team’s many papers and funded studies have all incorporated some variety of light scattering.

What exactly are these light scattering techniques, and when do you use them?
MALS directly measures the level of light scattering that occurs as a result of the molecular mass of a biopolymer, enabling the independent measurement of its properties in solution. The mass, and consequently the amount of scattered light, increases due to macromolecular interactions between two molecules, such as protein and protein or a protein and a nucleic acid. The phenomenon can be as simple as two monomers that form a dimer or more complex events like polymerization and protein aggregation.
Dynamic light scattering, or DLS, measures the fluctuations in intensity of scattered light that arise from a molecule’s Brownian motion. The signals are analysed to determine the translational diffusion coefficient and hence the molecule’s size, or hydrodynamic radius.
DLS and MALS have become day-to-day prerequisites for success in the study of biological macromolecules. For those using de-novo techniques like cryo-electron microscopy, X-ray crystallography, or NMR, routine analysis and batch-to-batch validation of recombinant preparations are critical steps towards successful structure determination.
For enzymologists characterizing the activity and inhibition of recombinant preparations, batch to batch validation is key to accurate findings and the assessment of lead targets. For a small angle x-ray and neutron scattering scientist like myself, it is almost impossible to reliably interpret experimental results without these key experimental measures.
I personally have performed several hundred SEC-MALS experiments here at Penn over the last decade in a wide array of investigations, including protein-nucleic acid interactions, protein-protein interactions in the context of RNA splicing, HIV biology and drug discovery, site-specific recombination and chromatin structure.
How does SEC-MALS work?
SEC-MALS is size-exclusion chromatography in line with multi-angle light scattering. In biochemistry and structural biology studies it is one of the most popular implementations of light scattering.
The samples of interest are passed through a standard chromatography column to separate the macromolecules according to their size. The eluent from this separation then enters into a multi-angle light scattering instrument where there are multiple angles of light scattering recorded every second. The differential refractive index detector can be used to directly determine the concentration of the macromolecule at each time point.
Molecular mass can then be determined from the light scattering and concentration data, without the need for any standard curves or theoretical extinction coefficients. The analysis is independent of retention time and any assumptions about conformation or column interactions.
Can you tell us more about your HIV research?
The HIV AIDS pandemic remains a major global public health problem with 37 million people worldwide currently infected. It is projected that 30 million people will be taking anti-retroviral regimens by 2020. However, treatment efforts are being hampered by the development of viral resistance to current antiretroviral drugs so there is a need for novel therapeutics.
FDA-approved anti-HIV drugs are strand transfer inhibitors (STIs) that block the integration of HIV DNA into the host genome to form a provirus. The newest generation of these compounds are now being optimized to counteract emerging drug resistance and improve drug half-life.
My primary area of research is the HIV integrase enzyme, which—among other things—catalyses strand transfer. Integrase is one of three enzymes encoded by the HIV genome. It is a 32-kilodalton, three-domain protein.
The most distinguishing feature of the recombinant HIV integrase protein is its propensity to self-associate and form oligomers, which makes its structure difficult to study. Although partial crystal structures are available, the full-length protein has eluded investigators worldwide for many years.
Image Credit:Shutterstick/BioMedical
How does SEC-MALS contribute to such research?
The application of small angle scattering techniques based on x-rays and neutrons, SAXS and SANS, to structural biology and solution biophysics has increased exponentially over the past 15 years. They provide rich information, and are among the key tools we use to learn about molecular structure.
In these approaches, a columnated beam of either x-rays or experimental neutrons is directed at the sample of macromolecules and the intensity of scattering is plotted as a function of the scattering angle.
Using a Fourier transform analysis, we can gain insight into the shape and spatial extent of the molecule. This information can then be directly compared with profiles of known atomic structures to confirm structure and composition in solution.
However, if a sample is even slightly contaminated or partially oligomerized, the interpretation of these results is greatly compromised. This is where characterization and quality control by SEC-MALS have been critical to the growth and application of small-angle scattering techniques, since SAXS and SANS tend to involve expensive beam time and travel.
Among other benefits, SEC-MALS is far more sensitive to the presence of large aggregates than SEC-UV. In the case of a 440 kDa, Lactocous Lactii Group II intron protein-RNA complex, it detected low levels of large aggregates not detectable by UV. As a result we performed an additional purification step, finally enabling successful SAXS measurements and determination of high resolution cryo-EM structures.
SEC-MALS is key to the success of study of HIV integrase host factor interactions using small angle x-ray scattering. It enabled us to screen out many mutated and truncated forms of recombinant integrase complexes, so we can obtain high quality data of the complex of interest. This was highlighted in our study of the interaction between integrase and host lens epithelium-derived growth factor (LEDGF), which is an important part of the viral life cycle.
Similarly, in our 2012 bifocal study, SEC-MALS analyses were critical to the characterization of a related retroviral integrase protein-DNA complex. We confirmed using SEC-MALS that the integrase protein binds to viral DNA ends with a stoichiometry of four integrase to two DNA. SEC-MALS enabled us to optimize buffer conditions and limit aggregation.
Image Credit:Shutterstock/Explode
Has SEC-MALS has been critical to the success of your research?
Absolutely. In our most recent structural studies of the interaction of HIV integrase with the complex new class of drugs known as ALLINIS, SEC-MALS along with other techniques provided the breakthrough needed to obtain the full-length protein structure for the first time. This in turn enabled us the investigate the protein-protein interactions that were revealed by our new structure.
The ALLINIs are potent allosteric inhibitors of HIV integrase that are now being evaluated in clinical trials. They were identified though study of the crystal structures of the LEDGF-integrase interaction. It became apparent that small molecules could block this interaction and prevent HIV replication very early in the process.
The LEDGF binding site on the integrase protein was identified from the structure we obtained with the help of SEC-MALS, and molecules that prevent this interaction from occurring were developed using structure-based design. Potential drug candidates were selected through screening for the ability to disrupt the binding of the host factor LEDGF to integrase.
What do your studies with ALLINIS show?
Our studies focus on two ALLINIS (GSK1264 and GSKOO2). Both compounds, share a common linkage connecting a substituted central aromatic ring system. And x-ray crystallography has shown that they both bind to the same site on the catalytic domain of integrase with high affinity.
Both molecules have potent late-replication effects that dramatically disrupt HIV particle organization and alter the distribution of morphology of the viral particles. This is linked to their ability to catalyse the aggregation of HIV integrase independently of temperature and concentration. Such aggregation was monitored by SEC-MALS and shown to require both the catalytic core domain and the C-terminal domain of integrase.
We wanted to know how these drugs worked at the structural level. What are the structural changes that lead to drug-induced integrase aggregation?
To discover this, we needed the structure of the enzyme-drug complex. Since the drug promotes the aggregation of an already marginally soluble protein, this was not conducive to obtaining an ordered crystal lattice for analysis by x-ay crystallography. Instead, we set out to characterize numerous point mutations of the complex using multi-angle light scattering, analytical ultracentrifugation, small-angle x-ray scattering (SAX) to identify which forms of the integrase protein were less responsive to the ALLINIs.
Image Credit:Shutterstock/Sciencepics
Using both SEC-MALS and sedimentation equilibrium analysis, we discovered mutations of integrase that existed solely as dimers. Drug-induced polymerization was greatly attenuated even at elevated drug concentrations. For the first time we were able to crystallize the full-length, intact protein complexed with an ALLINI.
We were able to solve the structure using a molecular replacement research model for the previously determined structure of the catalytic core domain and the C-terminal domain. Extensive protein-protein interactions were observed that have not been seen with any other integrase form. The C terminal domain from one dimer reaches across to the core domain of a neighbouring dimer, much akin to a hand gripping a ball.
Mutations of integrase that conferred resistance to ALLINIS fell into two categories. They either directly disrupted the binding interface or perturbed drug positioning preventing it from binding. By introducing specific integrase mutations, we were able to confirm our new understanding of this drug-stabilized protein-protein interface; again, using SEC-MALS to determine oligomeric forms, in combination with other techniques.
We have conducted a range of SEC-MALS studies to further explore the structure of the integrase-ALLINI complex. The most surprising finding, as underscored by our new crystal structure, is the anticipated polymerization and late replication effects. The ALLINIs were selected and designed to interrupt protein-protein interactions between LEDGF and integrase, yet our results show that the drugs very efficiently stabilize an open polymer of integrase.
These results together provide insights into drug design approaches for this new class of drugs, just entering clinical trials.
Where can our readers go to find out more?
To find out more please visit https://www.wyatt.com/library/webinars/applications-of-light-scattering-to-hiv-integrase-structural-biology-and-drug-discovery.html
About Professor Kushol Gupta
.jpg)
Dr Kushol Gupta is a Research Assistant Professor in the Department of Biochemistry and Biophysics at the Perelman School of Medicine, University of Pennsylvania. He also serves as a director of the Johnson Foundation of structural biology and biophysics core.
Dr Gupta’s research focuses on HIV integrase and the antiviral effects of small molecules that target this protein. The use of multiple biophysical techniques, including x-ray crystallography, small angle x-ray scattering, light scattering, and analytical ultra-centrifugation, provides a deeper understanding of the structure and function of HIV integrase, more effective screening of anti-HIV integrase compounds, and a complete picture of the therapeutic mechanism of potential drug candidates.