While the COVID-19 pandemic is devastating many regions, the world over, a new paper published in the Journal of Experimental Medicine in April 2020 describes the possibility that the critical or very severe cases of COVID-19 are due to the production of neutrophil extracellular traps (NET) due to hyperactivated immune cells.
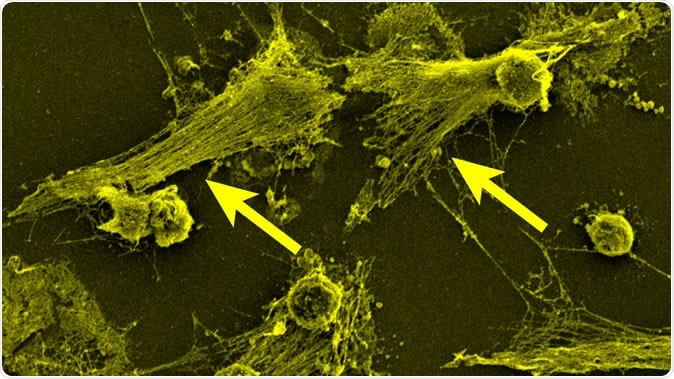
Part of the body's immune system, neutrophils detect bacteria and can expel their DNA (see arrows) to attack the bacteria with a gauzy web of DNA laced with toxic enzymes, called a NET. Image Credit: Egeblad lab/CSHL
The disease caused by the novel coronavirus dubbed SARS-CoV-2 has been called “the defining global health crisis of our time.” Indeed, it has caused more global panic than any event since World War II. Originating in China’s Wuhan city, in Hubei province, it has proved both highly infectious and, in up to 80% of cases, self-resolving.
The limitations of healthcare in almost every country affected by the virus have become evident. From a grievous lack of testing kits to inadequate numbers of ventilators and personal protective equipment, public health experts have found themselves strapped for resources at every turn. As a direct result, non-pharmacologic interventions such as social distancing, limitations on travel, contact tracing and quarantine, and cancellations of large social gatherings – and nation-wide lockdowns – have all become routine all over the world.
The crisis in this pandemic has to do with the 5% of cases who develop very severe or critical disease and often die of respiratory failure, sepsis, and breakdown. Researchers have been trying to discover the cause of such severe illness in this minority of cases.
The international consortium
Echoing the theme of solidarity in facing the pandemic, eleven medical research groups around the world have formed a consortium called the NETwork to investigate the potential role of NETs in the most severe cases of COVID-19. This includes organizations like the Cold Spring Harbor Laboratory, the Feinstein Institutes for Medical Research, and the Research Institute of the McGill University Health Centre (RI-MUHC).
What did the study find?
The researchers recorded that severe COVID-19 infection presents with acute respiratory distress syndrome, often seen in severe influenza. This includes the extensive appearance of lung inflammation and the production of tenacious mucus in the airways, clogging the airflow. This combination of issues causes widespread damage to the lungs. In addition, these patients experience the widespread formation of many clots in the body, a feature not common in other severe lung infections, and which makes it challenging to manage these patients. These are the critical cases, requiring mechanical ventilation, and among them, a significant percentage dies.
The reason, according to the NETwork, is that immune cells called neutrophils, which are among the most abundant of white blood cells, become overactive. These cells usually detect the presence of bacteria. To counter these invaders, the neutrophils shoot out part of their DNA content in a filmy web enriched with destructive enzymes. This is called a NET and can trap bacteria and digest them. However, excessive NET production is linked to ARDS, because of the accompanying damage they cause to the lungs.
What are NETs?
NETs are composed of segments of DNA along with globular protein domains in threadlike form. These gather to form larger, thicker strands that can form networks. The presence of NETs allows neutrophils to kill microbes outside the cell while minimizing host cell damage. The kill is accomplished by antimicrobial proteins like neutrophil elastase, cathepsin G, and histones, all of which bind closely to DNA. These are present at high concentrations in the NET, to attach to the entangled microbe, disarm it and destroy it. The NETs may also serve the purpose of walling off the bacteria to prevent its spread. Finally, the sequestration of these toxic proteases in the NET keeps them from damaging nearby healthy tissue.
The hypothesis is driven by the many similar features shared by ARDS, a condition known to be caused by NETs, and severe COVID-19. Says researcher Betsy Barnes, “As samples from patients become available, it will be important to determine whether the presence of NETs associates with disease severity and particular clinical characteristics of COVID-19.”
How NETs Harm Lungs
Tracing the role of NETs in disease and death
Another researcher, Mikala Egeblad, traces the first report of NETs back to 2004. However, she notes, they are still unfamiliar to most people, including many scientists. The NETwork includes people who have already been researching NETs in relation to other medical conditions. The clinical description of severe or critical COVID-19 disease rang alarm bells for these scientists, therefore.
Another scientist at the RI-MUHC, Jonathan Spicer, says NETs are also found to occur in patients with cancer or sepsis (the occurrence of infection in the bloodstream and seeding to multiple organs), conditions which are also associated with the presence of these microthrombi or small blood clots.
How is the finding useful?
The consortium is now analyzing the presence of NETs in COVID-19 samples. If, as they think, the overactivation of neutrophils leading to excessive production of NETs is responsible for severe clinical cases of COVID-19, it may indicate a new line of treatments to produce an improvement in their condition. For instance, they could explore the role and efficacy of medications used in other illnesses that involve NET, as well as in other conditions that result from neutrophil activity, such as cystic fibrosis, rheumatoid arthritis (RA) and gout.
Autoimmune diseases like RA could be the result of exposure of histone complexes outside the cell, stimulating antibody production. They also play a role in abnormal clot formation and are linked to the occurrence of stroke.
Such drugs could conceivably reduce NET activity in patients with severe COVID-19, limiting the need for mechanical ventilation and making healthcare available for a more significant number of patients with this condition.
Journal reference:
Betsy J. Barnes, Jose M. Adrover, Amelia Baxter-Stoltzfus, Alain Borczuk, Jonathan Cools-Lartigue, James M. Crawford, Juliane Daßler-Plenker, Philippe Guerci, Caroline Huynh, Jason S. Knight, Massimo Loda, Mark R. Looney, Florencia McAllister, Roni Rayes, Stephane Renaud, Simon Rousseau, Steven Salvatore, Robert E. Schwartz, Jonathan D. Spicer, Christian C. Yost, Andrew Weber, Yu Zuo, Mikala Egeblad; Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med 1 June 2020; 217 (6): e20200652. doi: https://doi.org/10.1084/jem.20200652