The need to understand the disease process of both mild and lethal COVID-19 as well as to evaluate how well various new therapies work has led to scientists looking at various animal models. A new study by researchers at the University of Iowa and published on the preprint server bioRxiv* in August 2020 report the utility of a mouse model that recapitulates several aspects of human COVID-19 and can reflect the antiviral efficacy of new drugs as well.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters cells via the ACE2 receptor, but wildtype mice lack this molecule. Thus, engineered K18-hACE2 mice have been developed for experimental purposes. These mice were developed to study SARS, which occurred in the form of severe encephalitis and mild pneumonia. However, SARS-CoV-2 in humans is more of a respiratory infection and also causes neurological signs such as anosmia, ageusia, coagulation defects, and endothelial damage. Heart muscle injury and pediatric multisystem inflammatory disease are also seen.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Significant Disease Following Infection
The current study focused on the clinical expression of SARS-CoV-2 infection in these mice. Intranasal inoculation showed a dose-related increase in weight loss and mortality, with the highest dose producing death of all infected animals, but not with the lowest dose.
When the viral RNA distribution was tested in different tissues by reverse transcriptase-polymerase chain reaction (RT PCR), at days 2, 4 and 6, they found that the lung was first affected and then the brain – both were centers of high replication, the lung more so than the brain. Other organs like the heart, spleen, and kidney were also affected, but replication was limited, perhaps indicating the bloodborne spread of the virus. The K18 promoter, which is responsible for hACE2 expression, mostly targets expression of the gene on tissues lined by epithelium, but also in nerve cells within the cortex and brainstem.
Widespread Progressive Lung Involvement
The viral N protein was found to be present extensively within the lung parenchyma on day 4 from infection, reducing by day 6. Signs of inflammation were widespread, with immune effector cells accumulated in the lung tissue, and some vascular clots. The alveolar septa were thickened by infiltrating inflammatory cells, dying cells, and proliferation of the alveolar epithelium.
The lung tissue also showed increased type I, II, and III interferons, as well as interferon-stimulated genes, several inflammatory interleukins, and TNF.
Immune Effector Cells in the Lungs
The injured lungs showed a progressive elevation of macrophages and monocytes, neutrophils, and T cells, both CD4 and CD8. These T cells are also seen in human lung disease caused by this virus, a common feature with this mouse model, along with the early antibody response detectable by day 6.
Brain infection in Some Mice
Most mice inoculated at the highest dose had an infectious virus in the brain on day 6 onward. Most affected areas were connected to the olfactory bulb, but not all. This indicates its essential role in enabling brain spread as well as the possibility that other routes of entry are also implicated. However, olfactory bulb infection could explain the anosmia and ageusia often seen.
Sinus and Nasal Epithelial Infection
Viral RNA was present in the nasal secretions of most mice, both genomic and subgenomic, indicating active replication. Viral antigen was also seen in the respiratory and olfactory epithelium at days 2 and 5. At such sites, cell death was seen, with sloughing and loss of cellular patterns. The ACE2 antigen was found on sustentacular cells present in the olfactory epithelium but lacking in the sensory neurons. This indicates that the former is primarily involved in infection. While this may not cause viral spread to the olfactory bulb, it could cause anosmia.
In fact, the researchers demonstrated anosmia, despite normal mobility and absence of brain infection. This indicates that nasal epithelial infection is the cause of the anosmia rather than spread to the brain.
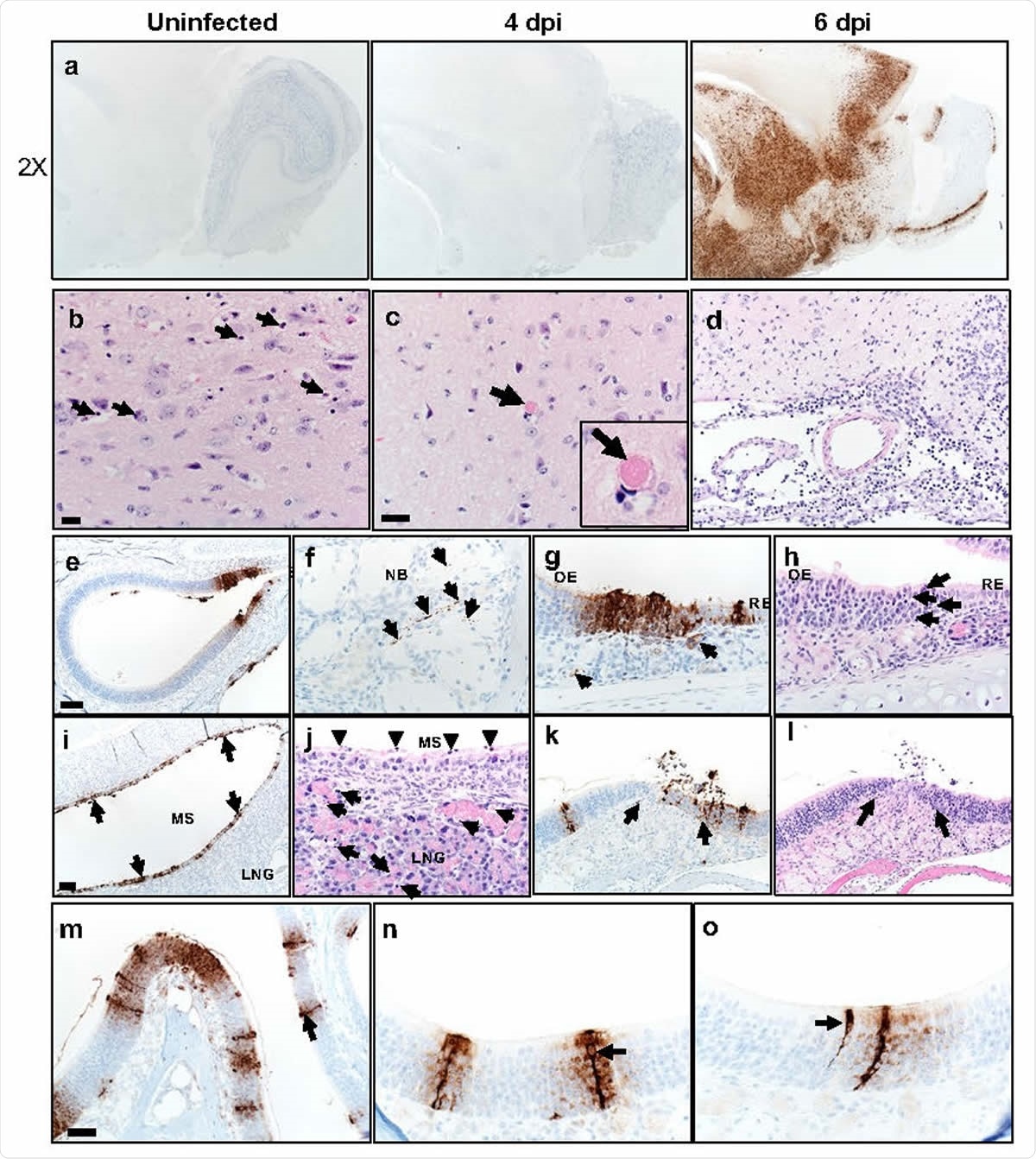
Some K18-hACE2 mice develop brain disease after SARS-CoV-2 infection. a. Brains were prepared from uninfected and infected mice and analyzed for SARS-CoV-2 by immunohistochemistry. b, c. Multiple sites of dead cells (arrows) characterized by cellular and karyorrhectic nuclear debris (b, arrows) and of thrombi (c, arrow and inset) in thalamus. Bar = 17 μm. d. Examination of meninges at day 6 p.i. revealed increased cellularity composed of degenerate cells and neutrophils and mononuclear cells. Cellular and karyorrhectic nuclei debris were also detected in the perivascular regions (200X). e-o. Nasal and sinus tissue were examined at days 2 (e-j) and 5 (k-o) p.i. eh. Olfactory epithelium (OE) was stained with anti-SARS-CoV-2 N antibody (e-g) or H&E stain (h). f. Nerve bundles (NB) subjacent to OE had punctate immunostaining (brown, arrows). g. Subjacent to sites of immunostaining for N protein (brown, black arrows), endothelial lining of vessels (left arrow) and bowman glands (right arrow) were occasionally positive for virus antigen (brown). h. Sites of N protein localization in OE had evidenced of cell death and cellular debris (arrows). Bar = 100 μm (e) and 25 μm (f-h). i-j. Maxillary sinus (MS) stained with anti-SARS-CoV-2 N antibody (i) or H&E (j). i. MS lining epithelium had extensive immunostaining for N protein (brown, arrows). j. ME epithelium had common sloughing and cellular debris (arrowheads). The lateral nasal glands (LNG) also had multifocal cellular and karyorrhectic debris (arrows). Bar = 75 μm (i) and 19 μm (j), respectively. k-l. Olfactory epithelium still had foci of SARS-CoV-2 N protein immunostaining at d. 5 p.i. (k, arrows, left) that was often localized near interface with respiratory epithelium. In these sites, there was cellular sloughing and loss of cellularity (arrows, right). 200x. m-o. Arrows point to “classic” morphology and strong labeling of sustentacular cells with expanding labeling of adjacent cells. Bar = 50 (m) and 25 μm (n, o).
Convalescent Plasma from Human COVID-19 Patients Prevents Severe Disease in Mice
Currently, convalescent plasma (CP) is being tried to treat patients with COVID-19. This is based on earlier studies showing the positive effects of CP in infectious disease if given early enough. The mice in the current study were given both diluted and undiluted CP 12 hours prior to infection. The results showed that undiluted CP prevented death, while when diluted 1:3, there was partial protection.
At 1:3 and 1:9 dilutions, the virus failed to spread to the brain with the use of CP, but the mice developed progressive lung disease. Lung changes were also markedly reduced by CP. Thus, this therapy did not prevent the virus from infecting the lung cells but did keep the virus from spreading within the lung.
When given at 5 hours after infection, undiluted plasma had a partially protective effect. However, when given at 1 day after infection, it failed to prevent anosmia even though the clinical disease was insignificant. This could be because some functions of antibodies depend on the specific species of production.
Thus, CP seems to enhance rapid viral clearance but not to prevent primary lung infection or damage to the epithelium of the nose, both respiratory and olfactory.
Mechanisms of Anosmia
The anosmia is probably due to sustentacular cell damage and not olfactory neuron injury. This means that inflammation underlies the anosmia and not direct nerve lesions.
Either the sustentacular cells are infected, preventing normal signals from passing from the olfactory neurons to the olfactory bulb. One mechanism is that the ionic balance of the olfactory neurons is disrupted by the failure of sustentacular cell support, due to infection or because of the disturbance in the normal organization of the olfactory epithelium, with loss of cilia from the olfactory neurons.
An alternative explanation is that the inflammatory cytokines secreted by the infected sustentacular cause inadvertent damage to the olfactory neurons. Of course, both mechanisms may be operative.
Implications
The current study shows a marked difference in the tissues affected by SARS and COVID-19. The first caused brain infection at low doses of virus intranasally administered. However, even higher doses caused brain infection in only some mice. As a result, the latter caused more obvious lung infection, but the first caused mostly brain damage involving the vital centers of the medulla, resulting in aspiration pneumonia.
All the mice died of the infection at high intranasal doses, mostly from lung but sometimes from brain disease. The study, therefore, reveals K18-hACE2 mice to be useful in uncovering the pathogenesis of various COVID-19 manifestations and for evaluating the efficacy of therapies.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zheng, J. et al. (2020). K18-hACE2 Mice for Studies of COVID-19 Treatments 1 and Pathogenesis Including Anosmia. bioRxiv preprint. doi: https://www.biorxiv.org/content/10.1101/2020.08.07.242073v1
- Peer reviewed and published scientific report.
Zheng, Jian, Lok-Yin Roy Wong, Kun Li, Abhishek Kumar Verma, Miguel Ortiz, Christine Wohlford-Lenane, Mariah R. Leidinger, et al. 2020. “COVID-19 Treatments and Pathogenesis Including Anosmia in K18-HACE2 Mice.” Nature, November, 1–8. https://doi.org/10.1038/s41586-020-2943-z. https://www.nature.com/articles/s41586-020-2943-z.