Researchers in Denmark and the Netherlands have conducted a study showing that sex and gender are not being adequately considered in clinical trials investigating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the coronavirus disease 2019 (COVID-19) pandemic.
The team of researchers from Radboud University Medical Center, Aarhus University and Copenhagen University says that although sex and gender are factors that influence the incidence of SARS-CoV-2 infection and the risk for COVID-19 mortality, only a small proportion of studies registered in ClinicalTrials.gov mention sex and/or gender as part of the recruitment criteria or analysis phase.
Sabine Oertelt-Prigione (Radboud University Medical Center, Netherlands) and colleagues say the study findings should be seen as a warning signal that researchers need to systematically consider sex and gender in the design of SARS-CoV-2/COVID-19 trials to ensure safe and effective therapeutic options for all participants.
A pre-print version of the paper is available in the server medRxiv*, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Sex and gender influence SARS-CoV-2 infection and mortality
Sex and gender differences are known to influence the incidence of SARS-CoV-2 infection and the risk of COVID-19-related death.
A growing body of evidence has pointed to an increased risk of mortality among males, compared with females, for example.
Oertelt-Prigione and colleagues suggest this could be attributed to sex differences in the immune response to infection or in specific features of the infection process.
To gain viral entry, SARS-CoV-2 binds to the host cell receptor angiotensin-converting enzyme 2 (ACE2), which is encoded on the X chromosome and interacts with a serine protease that is thought to be hormone-sensitive.
One recent study also highlighted the role that Toll-like receptor 7 (TLR7) plays in the innate immune response against SARS-CoV-2 infection, and this receptor is also encoded on the X chromosome.
Sex and gender should be included
The authors say that only including one sex in clinical trials and failing to disaggregate results by sex can lead to an increase in the incidence of unwanted side effects among the untested population as a result of overmedication and other factors.
“The investigation of sex differences could provide essential insights into COVID-19 pathophysiology and possibly aid the identification of effective interventions,” say the authors.
The study of gender differences is also warranted, they add. This multidimensional variable that describes identity, norms, and relations between people can affect access to testing, diagnosis, and healthcare and also influence the availability of social and economic support, says the team.
Failing to address the gender factor hinders the ability to reduce inequality in healthcare, promote preventive action, and change the course of infection.
Investigating the inclusion of sex and/or gender
Oertelt-Prigione and colleagues investigated the inclusion of sex and/or gender as an analytical variable in the SARS-C0V-2/COVID-19 trials currently registered on ClinicalTrials.gov.
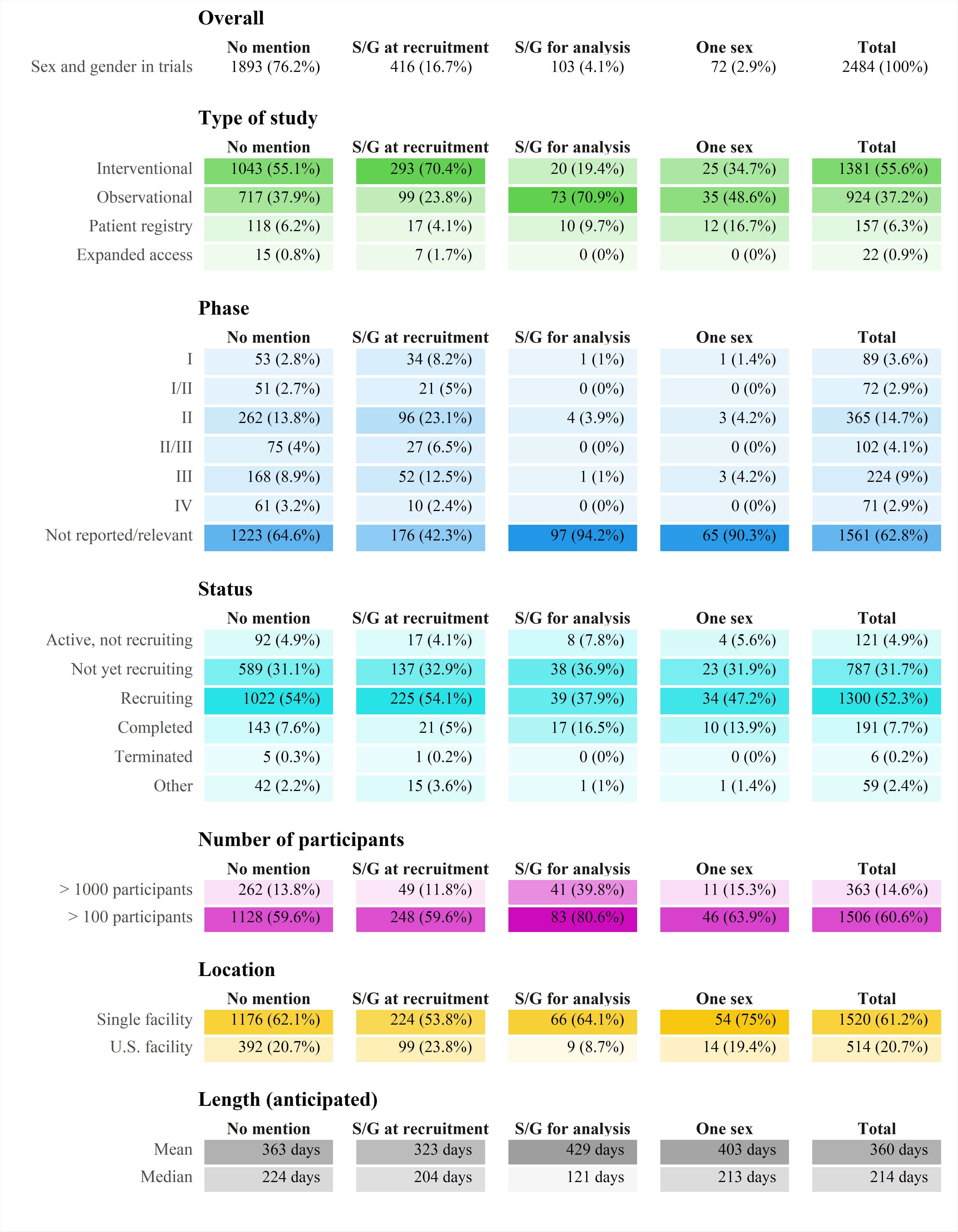
Characteristics of the included trials. Number of participants and length are mostly anticipated values. Only 83% of the registered trials record facility location. Abbreviation: S/G – sex/gender
The team identified 2,484 registered trails, 1,081 of which were observational, and 1,381 were interventional.
Of these registered trials, only 416 (16.7%) included sex and/or gender as a recruitment criterion or recorded variable, and only 103 (4.1%) mentioned sex/gender in the analysis phase.
Seventy-two trials (17.3%) focused only on one sex; 61 studies included females only, and 11 included males only.
Of the 1,381 interventional trials, only 20 planned to include sex as a variable in the analytical phase.
A PubMed search for trials published in scientific journals on June 20th retrieved 11 randomized controlled clinical trials. All of these trials reported on the number of males and females included, but none reported sex-specific analysis of disaggregated results by sex.
“This suggests that a lack of consideration of sex and/or gender upon trial registration will not be revised during trial execution, leading to a significant information gap,” writes the team.
Urging researchers to focus on sex-disaggregated analyses
The authors say that although sex is considered an essential determinant of mortality risk and immunologic responses to COVID-19, the clinical trials currently registered generally exclude sex as recruitment or analytical criterion.
“Given the biological relevance and the potential risks of unwanted side effects, we urge researchers to focus on sex-disaggregated analyses already at the planning stage of COVID-19 trials,” they write.
Gender is receiving even less attention
The authors say that even less attention is being paid to gender in the trials.
Only 191 (7.7%) of the 2,484 trials mentioned gender as a recruitment or analytical variable.
“Gender is, however, a relevant risk factor,” writes the team. “For example, recent reports highlight that infected healthcare workers worldwide are more frequently women and that women might be more affected by persistent symptoms after initial COVID-19 infection.”
Recommendations for future studies
The researchers emphasize that sex-specific analysis is not a particularly complicated process and that its exclusion may be the result of a lack of awareness rather than a deliberate decision.
“The presented data should be considered an alarming signal, which can still be addressed,” say Oertelt-Prigione and colleagues
“Sex and gender should be systematically considered in COVID-19 trial design to guarantee safe and efficacious therapeutic options for all patients,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources