A pilot study testing wastewater from buildings as a surveillance method for COVID-19 found it to be a feasible method for detecting positive cases in highly occupied settings like dormitories.
As the COVID-19 pandemic sweeps across the globe, several strategies have been put into place to prevent the spread, such as social distancing and wearing a mask.
However, maintaining a distance and always wearing a mask is difficult, especially in places like college dormitories, nursing homes, and prisons, where many people are gathered, mainly indoors. Entry screening and temperature checks have not contained infection spread effectively as people may be contagious even when they have no symptoms. This has led to a spike in cases in such settings.
Cases may be reduced in close-living settings by frequent testing and quickly isolating people who have tested positive and quarantining their contacts. However, regular testing is expensive, requires testing personnel, and may lead to anxiety among occupants.
Stool has large amounts of mainly non-viable SARS-CoV-2 virus, and the virus is shed for many weeks after the infection. This observation has led to a great interest in testing wastewater for monitoring the infection at a community level.
This could be very useful for monitoring infection in buildings and potentially serve as early surveillance of occupants. Wastewater testing could also help determine when and where interventions are required, without the need for large-scale and frequent individual testing.
Wastewater testing protocols
In a new study published on the preprint server medRxiv*, researchers investigated if testing pooled wastewater samples frequently could be an efficient method of monitoring COVID-19 infections in a building.
The researchers collected wastewater samples from a hospital building and dormitories at the University of Virginia, and wastewater from a water treatment plant in Charlottesville, Virginia, and from a private residence.
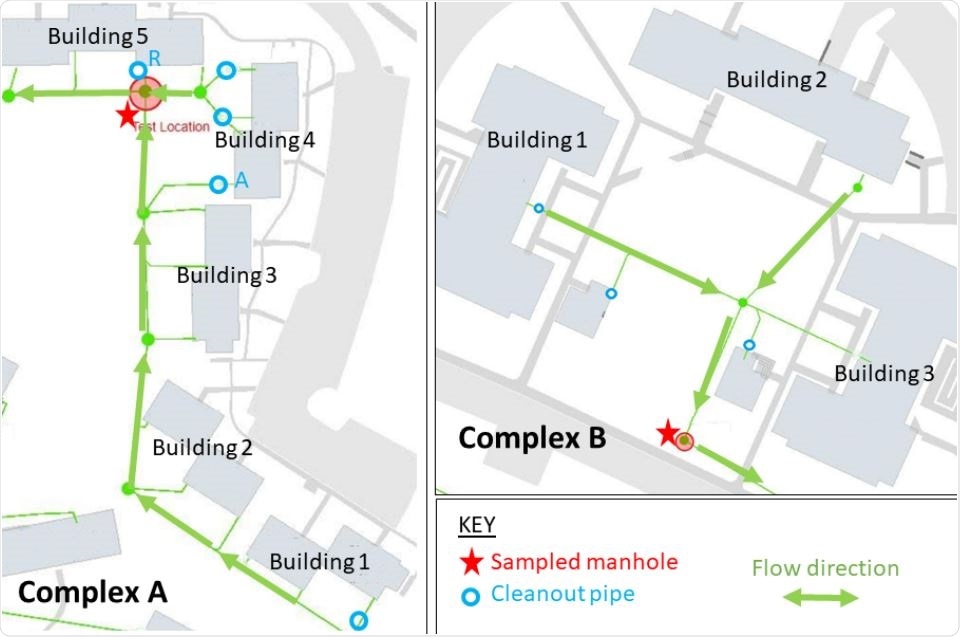
Maps of UVA dorms in Complex A (left) and Complex B (right). Red stars denote each sampled manhole. Arrows indicate flow directions. Cleanout valves labeled “A” and “R” denote secondary testing locations (via cleanout pipes) for selected buildings in Complex A.
The team had to ensure that the samples collected were connected to the waste coming COVID-19 unit in the hospital. For this, they used a dye to trace the flow of wastewater and, after a couple of tries, decided on a location that was connected to patient rooms in the hospital building.
From the collected samples, the authors used four different methods to concentrate the virus particles and recover viral RNA. They extracted RNA using commercial RNA extraction kits and tested for SARS-CoV-2 using RT-PCR.
They found that SARS-CoV-2 was detectable in wastewater from the buildings and the water treatment plant. The ultracentrifugation concentration protocol showed the least inhibition and more positive results. Water from the water treatment plant did not need any concentration protocol as the solids settled down by gravity.
Wastewater testing is feasible for detecting SARS-CoV-2
To validate the results of the wastewater analysis, the team compared the cycle threshold, Ct, values to the known case counts in the buildings. They found a sensitivity of about 95% and a specificity of about 100%, except when there was a recovered patient, which they missed, and the specificity dropped to about 50%. Thus, the method cannot differentiate between the presence of a new contagious patient and a recovered patient with continued viral shedding.
After analyzing the results, the team found that the number of occupants in a building plays a role in the ability to detect the virus in wastewater. There was one instance when a positive case was not detected in a building with 15 people. This may be because low water use meant less sample collection.
Thus, the authors write this method may be more suitable for larger buildings with more than 20 people.
Another important factor in detecting SARS-CoV-2 in wastewater is when, after collection, sample processing, and testing was done. They found that detection of SARS-CoV-2 decreased slightly the longer it took for the samples to be processed and tested.
So, it is critical to prevent RNA degradation after sample collection, for example, by refrigerating or storing the samples in ice until processing. Long travel times of the samples to testing facilities may reduce viral RNA detectability and could decrease the usefulness of wastewater testing as a surveillance method.
For sample concentration, the authors found ultracentrifugation to be the best. However, it requires costly ultracentrifuges, and it is a batch process, which may not be very convenient when testing a large number of samples.
Another key aspect of wastewater samples is effective RNA extraction. Both the two commercial kits the team tested showed inhibition. Hence, RNA extraction protocols need to be carefully tested and optimized to ensure good detection sensitivity.
With the specificity and sensitivity detected, the authors believe the method can be used to detect cases in buildings even when the number of cases is small or in asymptomatic cases. Once there is a positive result using this method, individual testing of all building residents can be performed for detecting individual cases.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources