Over 54.76 million people worldwide have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative pathogen of coronavirus disease 2019 (COVID-19). So far, over 1.32 million people have lost their lives to the virus in one of the greatest pandemics in recent memory.
As yet, no effective and safe therapeutic medications for COVID-19 or preventative vaccines against SARS-CoV-2 infection have been developed and rolled out for general usage. Therefore, the search continues to develop effective therapeutic options to treat COVID-19, while vaccines against its causative pathogen undergo clinical trials and await regulatory body approval.
United States-based researchers Camille Celeste Go, Krunal Pandav, Marcos A. Sanchez-Gonzalez and Gustavo Ferrer have published a report exploring the potential role of Xylitol and Grapefruit seed extract (in the form of a nasal spray) in treating COVID-19. Their study titled, “Potential Role of Xylitol Plus Grapefruit Seed Extract Nasal Spray Solution in COVID-19: Case Series,” was released in the open-access journal Cureus.
Background
The authors of the study explain the significant impact of COVID-19 on the healthcare systems of many countries around the world. The dearth of therapeutic options has compounded these pressures, the team writes.
Nasal epithelium and drug targets
They explain that angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) are two potential drug targets. The ACE2 receptor acts as the binding site where the virus attaches to enter into the host cells. The TMPRSS2 also plays an important role in viral entry into the cells. These are both present abundantly in the bronchial epithelium and alveolar type II epithelium cells, as well as the epithelial lining of the nose.
Previous studies
Earlier studies have shown that nasal sprays could be a good option in both preventing SARS-CoV-2 infection and treating it.
Prior studies have also emphasized how viral shedding occurs mostly from the nose and nasal cavity, which puts healthy individuals in contact with those infected at greater risk.
Xylitol and grapefruit seed extract
The researchers identified two agents: xylitol and grapefruit seed extract (GSE), which, when administered intranasally in the form of nasal sprays could help ameliorate COVID-19 symptoms in patients.
Xylitol has had demonstrable antiviral effects in labs against several viruses, including avian influenza virus (AIV), Newcastle disease virus (NDV), infectious bursal disease virus (IBDV), the team explains.
They used the xylitol-GSE nasal spray for a duration of seven days in COVID-19 patients and presented a case series of three different patients to chart its impact on their bouts of the disease.
Case 1
A 16-year-old female tested positive for COVID-19 on July 7, 2020. She was a non-smoker and had a history of iron deficiency anemia but no other comorbidities.
Their symptoms of COVID-19 were:
- Sore throat, dry mouth
- Nasal congestion, runny nose
- Productive cough with yellow sputum
- Anosmia, and ageusia (loss of taste)
After testing positive, she was enrolled in the study and instructed to take the nasal spray twice per nostril four times a day every six hours for seven days. The course of her disease was as follows:
- Day 1 - the patient had a stuffy nose, anosmia, ageusia, tiredness, cough, stuffiness, and congestion with normal pulse and oxygen saturation and temperature. She had mild symptoms on the Symptoms Assessment Score (SAS)
- Day 3 - she could smell strong substances. There was an improvement in their cough, and they showed normal levels of c-reactive protein (CRP) and d-dimer
- Day 7 - she showed improvement in overall symptoms with the absence of a cough, congestion, and stuffiness and reduced weakness and ageusia.
- Day 7 - she tested negative for COVID-19
- Day 14 - she returned to baseline health with no symptoms
Case 2
A 60-year-old male tested positive for COVID-19 on July 7, 2020. The patient had a history of leukemia, presently in remission. They were a heavy smoker and occasionally consumed alcohol.
Their Symptoms at the start of the condition included:
- Sore throat, dry mouth
- Sneezing, nasal congestion, runny nose
- Anosmia and ageusia
- low-grade fever at 101 Fahrenheit (F)
After testing positive, the patient was given nasal spray four times a day every six hours for seven days. The course of his disease was as follows:
- Day 1 - the patient had a stuffy nose, sneezing, congestion, sandy and watery eyes. He had an oxygen saturation of 97% at room air and a pulse rate of 86 beats per minute. They also had anosmia and fever (101 F). Overall symptoms were rated mild on the SAS.
- Day 2 - weakness and productive cough, with awakenings at night time due to coughing episodes. There was ageusia. No fever and oxygenation and pulse were stable.
- Day 3 - their symptoms improved with only sandy eyes, anosmia, and ageusia
- Day 4 - their smell slowly returned, and CRP and d-dimer tested normal.
- Day 7 - they showed 70 to 80 percent improvements in anosmia and other symptoms of tiredness and ageusia. No fever since day 2.
- Day 7 - they tested negative for COVID-19
- Day 14 - they returned to baseline health with no symptoms
Case 3
38-year-old male tested positive for COVID-19 on September 26, 2020. The patient had a body mass index 30 and was a non-smoker. Their symptoms were:
- Flu-like symptoms
- Night sweats
- Nonspecified fever
- Chest X-ray showed reduced lung volumes
After testing positive, the patient was given the nasal spray to spray twice per nostril four times a day every six hours for seven days. The course of his disease was as follows:
- Day 1 - the symptoms included runny and stuffy nose, tiredness, productive cough, nasal congestion and diarrhea. On examination, oxygen saturation was 94 percent, and there was no fever. Overall symptoms were rated mild.
- Day 4 - their CRP and d-dimer were tested to be normal
- Day 7 - symptoms reported were tiredness and a cough, which had improved since onset. No fever since onset.
- Day 7 - RT PCR result was negative.
- Day 14 - no symptoms and return to baseline health
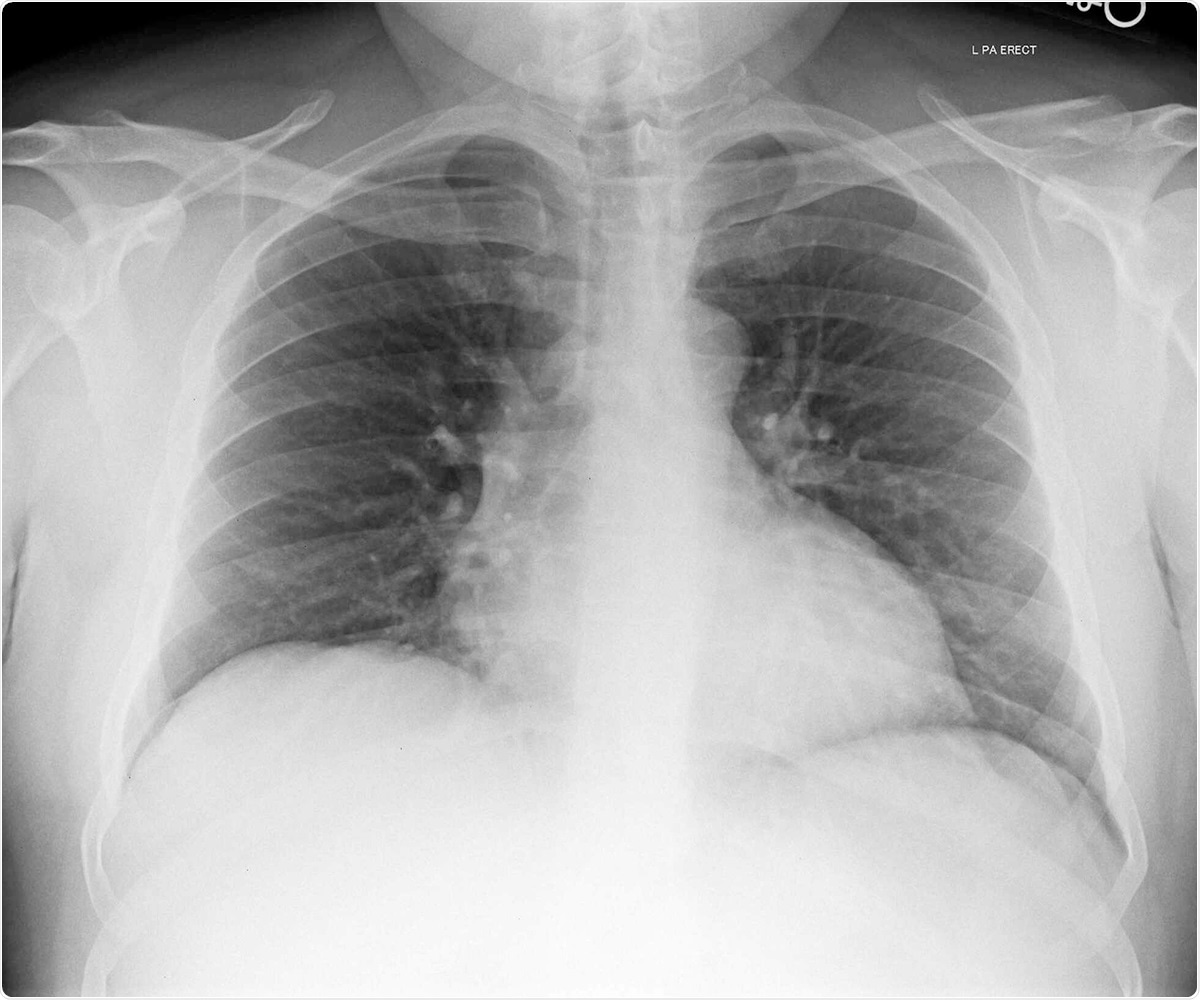
Chest X-ray image of patient 3. Image Credit / Original Article.
Conclusions and implications
The three COVID-19 patients presented with mild-moderate risks and mild symptoms. They were given the intranasal nasal spray along with other adjuvant supportive treatments. The patients showed, “rapid clinical improvement and shortened time to negativization on repeat intranasal swab test via PCR.”
The spray was found to be safe, and the authors suggest that this spray could be “a potential adjunct treatment option in mild-moderate COVID-19 cases.”