It is widely known and recognized that COVID-19: i) attacks some mammals but not others; and, ii) the attack is more severe in the case of the old and sick, than in the young and healthy.
Hitherto, no underlying explanations are known for these disparities in the infectivity of SARS-CoV2 and in the severity of the disease it causes, COVID-19. We set out to seek an explanation of these disparities.

Elderly man in face mask. Image Credit: Yuganov Konstantin/Shutterstock.com
What groups are more vulnerable to COVID-19?
COVID-19 hits the elderly and people with underlying conditions more severely than others.
How have you used different animals in your research?
We assembled a multidisciplinary team of scientists which includes Professors Jaswinder Singh, Rajinder Dhindsa (McGill University), Baljit Singh (University of Calgary) and Vikram Misra (University of Saskatchewan) to explore the infectivity of SARS-CoV2.
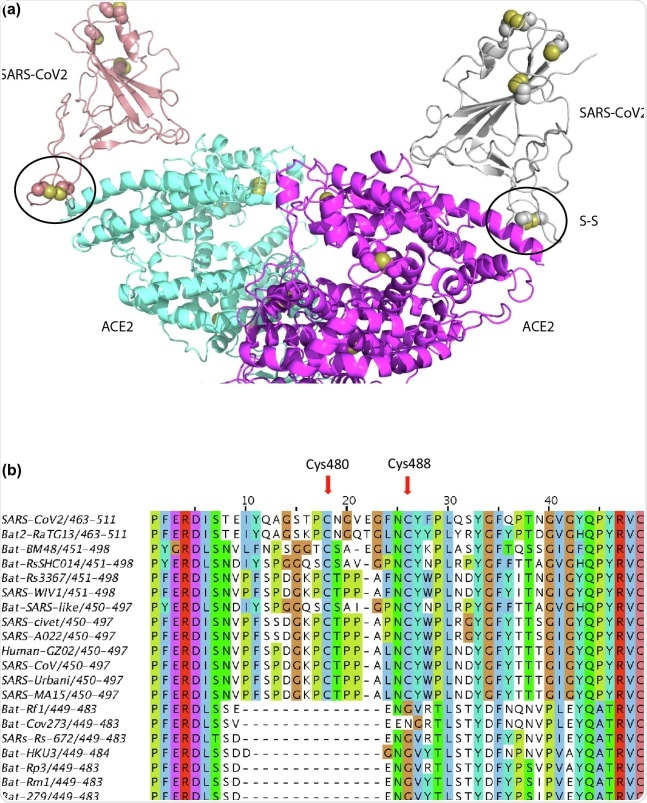
We retrieved the amino acid sequences of spike proteins from 20 different coronaviruses that all use the same host receptor and cause disease in various organisms.
We also aligned amino acid sequences of angiotensin-converting enzyme 2 (ACE2) receptors from 16 vertebrate coronavirus host species including humans, dogs, cats, horses, ferrets, pigs, and cattle.
How does COVID-19 infect cells?
To initiate infection, two spike proteins of coronavirus bind to the membrane-anchored dimer of the ACE2 receptor of the host cell.
More specifically, the receptor-binding domain (RBD) of spike protein binds to the peptidase domain of the membrane-anchored ACE2 receptor of the host cell.
Once inside a host cell, the virus hijacks the cell’s metabolic machinery to make many copies of itself, releases from the cell, and spreads to infect other cells.
Ribbon representation of the complex of the receptor binding domain (RBD) of the surface spike glycoprotein (S-protein) of SARS-CoV2 bound to the extracellular domain of the human ACE2 receptor. Image Credit: Jaswinder Singh
What did you discover about why certain animals are vulnerable to COVID-19 infection and others are not?
We know that the virus can infect humans, cats, dogs, and ferrets but not cows and pigs. In analyzing the proteins and their amino acid building blocks, we found that animals susceptible to the virus have a few things in common.
Mammals like humans, cats, and dogs have two cysteine amino acids that form a special disulfide bond between them held together by an oxidizing cellular environment. This disulfide bond stabilizes an anchor for the virus to latch on to.
In the case of animals resistant to the virus, like pigs and cows, one of these two cysteine amino acids is missing, and the disulfide bond cannot be formed. As a result, the virus cannot anchor on to the cell.
What does your research suggest is behind the elderly and those with underlying health conditions being more vulnerable to infection with increased severity of COVID-19?
The cellular environment becomes more and more oxidizing with aging, disease, and stress, which favors the disulfide bond formation by the aforementioned two cysteines. The infectivity and severity of COVID-19 are affected by whether both of these two cysteines are present and whether they are in reduced (sulfhydryl state) or oxidized (disulfide state) state, our hypothesis may be called the “Redox Hypothesis”.
Our analysis suggests that greater cellular oxidation in the elderly or those with underlying health conditions could predispose them to more vigorous infection, replication, and disease.
How could this research lead to new treatments and therapies?
Preventing the anchor from forming could be the key to the unlocking of new treatments for COVID-19. The potential role of redox changes in the cellular environment in the virus infection and the resulting severity of COVID-19 disease is highly unique.
One strategy could be to disrupt the oxidizing environment that keeps the disulfide bonds intact. Antioxidant supplements could alleviate such cellular conditions and thus decrease the severity of COVID-19 by interfering with the entry and proliferation of the virus in host cells after establishing infection.
Improving the expression of thioredoxin reductase may be a promising therapeutic target for the development of novel and effective treatment strategies, as noted for other diseases.
Proposed Trx-dependent redox model for interaction of SARS-CoV2. Image Credit: Jaswinder Singh
How will your theories need to be tested before conclusions can be made?
Interaction of normal and mutated versions of SARS-CoV2 with human cell lines could be observed in different redox environments. In addition, some other proteins in the vicinity of the animal receptor ACE2 have been recently shown to facilitate the entry of the virus into the host cell.
We are studying these proteins to see if they behave according to our redox hypothesis. CRISPR technology could be used to edit protein sequences to test our theory.
What are the next steps for your research?
We are also looking into other proteins that may be facilitating the entry and replication of the virus.
We aimed to apply CRISPR-based technologies to target and modify spike protein and receptors of appropriate model animals to elucidate the underlying mechanisms.
Where can readers find more information?
About Dr. Jaswinder Singh
Dr. Jaswinder Singh is currently serving as an Associate Professor in the Department of Plant Science, McGill University, Canada. After completing his Ph.D. from CSIRO, Canberra Australia, he did his postdoctoral studies at the University of California, Berkeley. His research focuses on genomics and biotechnological approaches. His findings have shown for the first time the reversal of epigenetic silencing.
Currently, his team is working on cysteine-rich proteins and their role in the redox regulation of transcription factors and protein-carbohydrate interaction. He is the Director of the multi-institutional NSERC-CREATE program on the “Genome Editing for Food Security and Environmental Sustainability”. He has published over 50 research articles and delivered over 60 invited talks in renowned academic institutes and international meetings.
To date, he has trained more than 50 researchers, which include undergraduate, technicians, graduate students, and post-doctoral fellows. He has served on various executive positions in different scientific societies, notably as the President of the Canadian Society of Agronomy (2018-19), membership officer of the Canadian Association of Plant Biotechnology (2013-14), member of the International Affairs (2012-15), and the Enid MacRobbie Corresponding Membership award committees (2020-2023) of the American Society of Plant Biologists.
He is also an acclaimed researcher, receiving accolades such as the prestigious C. D. Nelson award in 2018 for his outstanding contribution to plant biology.