The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has highlighted the shortcomings in emergency response globally and has driven a massive demand for in vitro diagnostic testing technologies.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Quantitative real-time polymerase chain reaction (qPCR) technology is being widely used as a diagnostic test for SARS-CoV-2 detection, but it does not optimally fulfill the large-scale diagnostic needs of this unprecedented global pandemic. Long turnaround times, complexity, supply chain shortages, and high cost are some disadvantages of RT-qPCR that decrease its efficiency and hampers access to testing.
Many countries are adapting to and developing molecular technologies to overcome these shortcomings. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) technology is one of them and may be a viable alternative to qPCR technology in addressing the diagnostic needs of the COVID-19 pandemic.
The RT-LAMP technique and its principle
LAMP is a detection technique that works on the same nucleic acid amplification principle as PCR. However, LAMP is an isothermal technology and does not need thermal cyclers, which cost several thousand dollars. Hence, RT-LAMP uses minimal equipment compared to RT-qPCR and is a better alternative to currently used testing methods.
LAMP testing requires at least 4 (and up to 6) different primers, of which 2 contain a self-complementary region that produces a perpetual single-stranded loop and 2 target the region in the single-stranded loop structure. Thus, LAMP can produce detectable amounts of DNA in almost the same time as PCR but at one single reaction temperature. DNA detection can be achieved via different methods such as turbidity or by using a fluorescent dye.
Earlier detection efforts have focused on using colorimetric detection of amplification products using a pH dye, or a magnesium indicator. The color changes produced by these dyes, though adequate, have limited visible contrast and can often be ambiguous.
LAMPShade Violet offers better visual clarity and limits ambiguity
In order to address the above issues, researchers from the US recently used the new pH dye LAMPShade Violet (LSV) in RT-LAMP. LSV has better visual contrast between high and low pH and a stronger inflection point, both of which enable easy interpretation of results and reduces ambiguity. Their study has been published on the preprint server, medRxiv*.
For the detection of SARS-CoV-2, the researchers used saliva as a respiratory specimen instead of nasopharyngeal swabs. Saliva sampling is much easier compared to collecting nasopharyngeal swabs. Previous studies have confirmed the compatibility of saliva with RT-LAMP testing even in the absence of RNA purification. The team added an inactivation step that normalizes the pH, inactivates RNases, and releases RNA. However, since saliva is heterogeneous in different individuals, a one-size-fits-all inactivation strategy is not possible.
Our purification optimization results also suggest several unexplored directions for future saliva-based nucleic acid diagnostics.”
So, they optimized an inactivation protocol with better success across heterogenous saliva samples combined with LAMPShade Violet. They developed a 10-minute RNA purification protocol from saliva and named it magnetic bead protocol SalivaBeads. They also developed a magnetic stick (MS), StickLAMP, that offers reliable bead-based RNA purification and simple and low-cost access to testing from saliva specimens.
"MS also has several advantages over the other traditional method of bead separation, magnetic rack purification.”
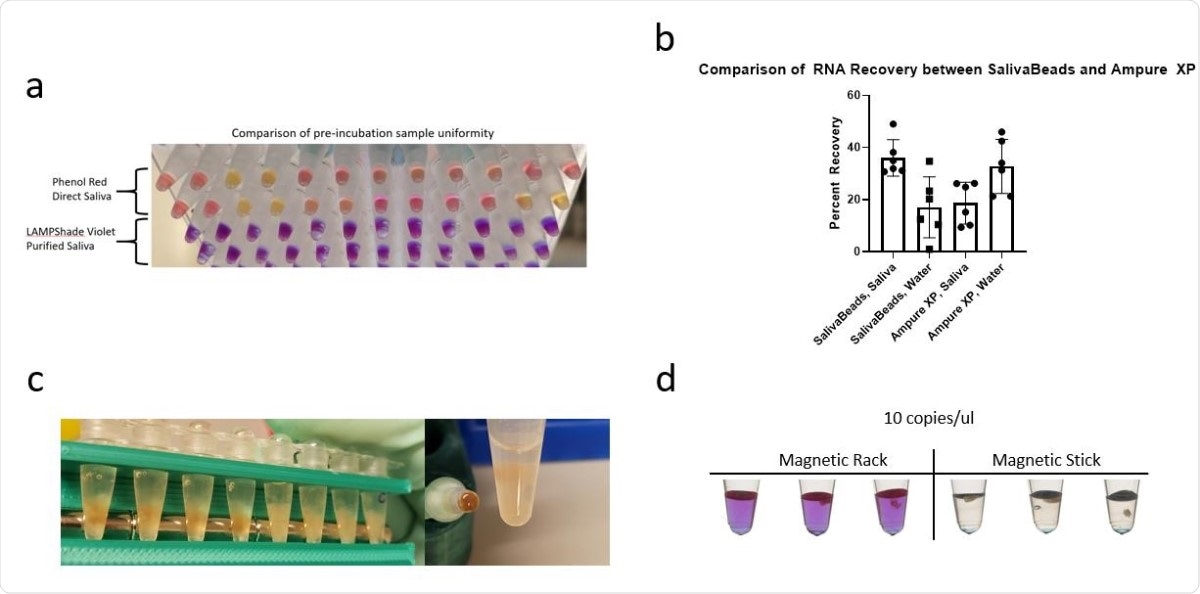
Several features of SalivaBeads purification. (a) A comparison of pre-incubation sample color uniformity in 12 samples, with SARS-CoV-2 and Actin reactions side by side. Direct input of inactivated saliva in a commercial Phenol Red-based reaction is compared to a SalivaBeads-purified RNA input into our LAMPShade Violet-based reaction. (b) A comparison of SARS-CoV-2 RNA recovered from contrived saliva samples by N1 qPCR, measured by number of copies recovered by qPCR divided by number of copies contrived into saliva. (c) A visual comparison of debris bound to magnetic-beads when isolating beads by magnetic rack (left) or a magnetic stick (right). (d) A comparison of sensitivity between SalivaBeads purified via magnetic rack (left) and magnetic stick (right), by RT-LAMP with LAMPShade Violet.
Low-cost protocols ideal for low-resource areas struggling to contain spread of SARS-CoV-2
According to the authors, the SARS-CoV-2 detection protocols they developed are cost-effective, at less than $5/test without factoring in labor and sample pooling, and they provide better scalability compared to existing protocols without affecting the sensitivity of the tests. The authors added that the new protocols are ideal for schools or workplaces with a small number of students or employees in the range of less than 10 to the low thousands. The low cost of the new protocols makes them suitable for low-resource and underserved environments that have been disproportionately affected by the SARS-CoV-2 pandemic.
The minimal equipment requirements and low cost also make them well-suited for low resource environments, which still might be able to mount a medium complexity CLIA lab.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Albert Dayuan Yu, Kristina Galatsis, Jian Zheng, Jasmine Quynh Le, Dingbang Ma, Stanley Perlman, Michael Rosbash. (2021) Development of a saliva-optimized RT-LAMP assay for SARS-CoV-2. medRxiv preprint server. doi: https://doi.org/10.1101/2020.12.26.20248880, https://www.medrxiv.org/content/10.1101/2020.12.26.20248880v1
- Peer reviewed and published scientific report.
Yu, Albert D., Kristina Galatsis, Jian Zheng, Jasmine Quynh Le, Dingbang Ma, Stanley Perlman, and Michael Rosbash. 2021. “Development of a Saliva-Optimized RT-LAMP Assay for SARS-CoV-2.” Journal of Biomolecular Techniques : JBT 32 (3): 102–13. https://doi.org/10.7171/jbt.21-3203-005. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8730519/.