Vaccines are thought to be the best solution for controlling and eradicating the currently circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), thus bringing the coronavirus disease 2019 (COVID-19) pandemic to an end. However, this hope is threatened by the repeated and rapid emergence of new variants that may be resistant to neutralization by vaccine-induced antibodies.
Modeling vaccination vs. transmission
The researchers used a simulation model implementing vaccination rates, as well as changes in transmission rates similar to those resulting from behavioral changes and non-pharmaceutical interventions. The research used the conventional SIR (susceptible-infected-recovered) model of disease transmission, which included additional states. These included the number of people vaccinated, those infected with resistant strains, or both together.
Typically, viral transmission occurs in waves during a pandemic, with the change in trends being brought about by factors such as environmental shifts, government policies, and individual behavioral changes. Thus, a period of high viral spread is followed by a period of low transmission, reflected by high or low effective reproduction numbers, respectively. This was mirrored in the values used.
The model also included a stochastic or random phase to reflect the influence of widespread genetic drift in the early stage of transmission of the resistant strains. The researchers modeled the phases as follows: the emergence of a resistant vaccine strain, its establishment, the point at which the number of resistant infections crosses 1,000, and finally extinction.
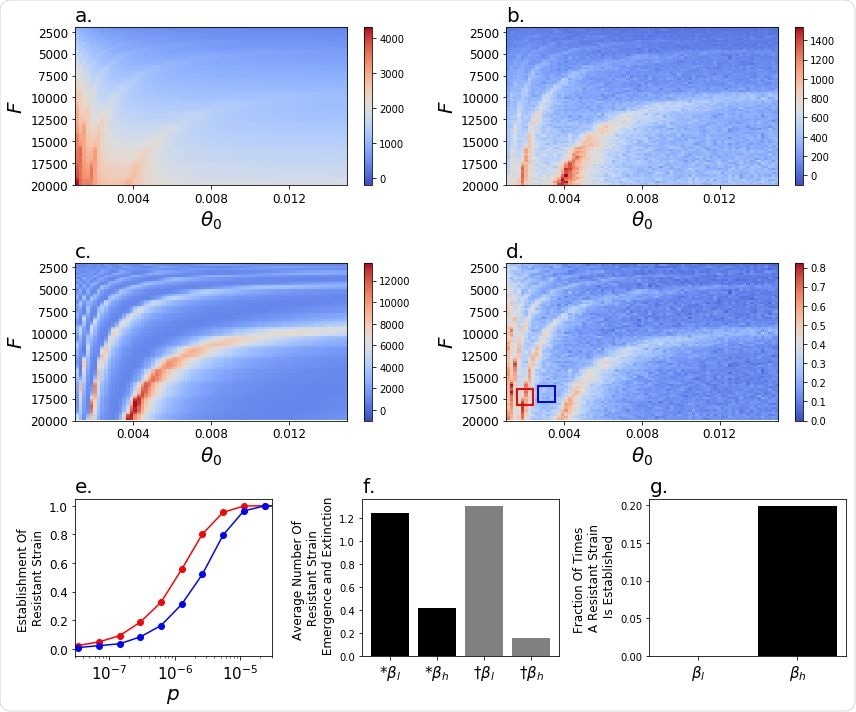
Impact of the rate of vaccination and initiation of low rate of transmission on model dynamics. The cumulative death rate from the a , wildtype and b, resistant strains, c , the number of wildtype-strain infected individuals at t v60 , the point in time when 60% of the population is vaccinated and d , the probability of resistant strain establishment, for p=10 -6 . e , The probability of emergence of the resistant strain as a function of the probability of emergence, p, in the parameter ranges of θ and F indicated in corresponding colour boxes in d . f, The average number of times of 8x10 6 simulation runs during which a resistant strain emerges (black) or goes extinct (grey) during periods of low (β l ) or high (β h ) transmission for p = 10 -6 . g, A resistant strain was never observed to establish during periods of low transmission (β l ) for p = 10 -6 .

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
High transmission favors the establishment of resistance
With high transmission rates, such as an effective reproduction rate of 2.5, even with no selection advantage for the resistant strain over the wild-type virus, the mutation will probably not be lost from the population. The model suggests that the resistant strain is established only during periods when the virus is spreading rapidly.
With a low rate of transmission, the effective reproduction number being below 1, the chances of extinction of the resistant strain are high because of genetic drift.
This is supported on theoretical grounds since high and low transmission periods may give rise to resistant strains. However, extinction is far more probable during periods of low transmission.
Time of establishment of vaccine-resistance
When the vaccination cycle overlaps with the period of the transmission cycles, complex interactions may occur. In one scenario, if a high number of infected people overlaps with a high number of vaccinated people, two consequences are possible.
Firstly, the high transmission rate prevents the extinction of the resistant strain, which might otherwise occur due to genetic drift. Secondly, the high vaccination rates allow the resistant strain to be selected over the wild-type virus.
The growth advantage conferred on the former allows it to be established when the vaccination campaign has achieved very high coverage. The most significant probability for this event is thus when the number of vaccinations and the number of infections and transmission rates, are all high.
The emergence of the resistant strain is thus frequently predicted to occur when 60% of the population is vaccinated. This is the period at which interventions are necessary to reduce the probabilities of the establishment of vaccine resistance.
Risk factors
The three factors that favor the emergence and persistence of vaccine resistance in the SARS-CoV-2 lineages currently in circulation are the high probability that such a resistant strain will emerge; the presence of many infected individuals; and low vaccination rates.
However, the analysis also shows, unexpectedly, that the establishment of a vaccine-resistant strain is most likely after the vaccination of a large proportion of the population in the presence of high transmission rates. Indeed, earlier data from the influenza pandemic bear out this conclusion.
Playing catch-up with the virus
The researchers comment on the high likelihood that as vaccine coverage increases to high levels, public policy will probably, out of necessity, shift to relaxed norms of social and economic interactions, approaching pre-pandemic levels. This is all the more likely once high-risk groups are protected.
The researchers point out that this is highly undesirable, however, with the right response being the implementation of strict social distancing at this period to extinguish the circulation of resistant strains. Otherwise, they warn, “the establishment of a resistant strain at that time may lead to serial rounds of resistant strain evolution with vaccine development playing catch up in the arms race against novel strains.”
Given that as of now, over 108 million individuals have been infected, with about 14 days of infection per individual, there have been ~1010 days of infected individuals. The probability that a resistant strain will emerge in one individual-infection day is about one in 105 to 108, making it a distinct possibility.
Another known, though uncommon, source of virus variation with resistance to common neutralizing antibodies is the prolonged incubation of the wild-type virus in an immunocompromised individual, with long periods of viral shedding. The existence of such probabilities seems to make it inevitable that a partially or fully resistant strain of the virus will emerge and become established.
What are the implications?
Using three parameters, namely, the probability of emergence of a resistant strain, the speed of vaccination, and the beginning of low-transmission periods, they explored the probability that a resistant strain could be established.
The close resemblance of this process to that by which a beneficial allele survives in a multiplying population led them to consider the problem in terms of population genetics. This shows that if the wild-type and resistant strains spread at equal rates, the chances of extinction of the resistant strain from the population depend on the transmission rates.
Two possibilities must be kept in mind while framing policies at such a time: firstly, high-risk group vaccination, followed by a population vaccination drive coupled with unrestricted viral transmission among the rest of the population, is a recipe for the establishment of vaccine-resistant strains, making vaccination a futile effort.
Secondly, if vaccinated individuals shed all caution while interacting in public with others, the resistant strain may spread at high rates.
To forestall this event, targeted efforts must be made to prevent viral transmission as far as possible towards the close of the vaccination period. This would take advantage of the efficacy of current vaccines at this time. A strict lockdown for a limited period would keep resistant strains that are just emerging to become extinct as they cannot circulate in the rest of the population and are subject to genetic drift.
Extensive and strict testing, contact tracing, genome sequencing and restricted travel may also be considered during this short period to increase the vaccine's efficacy. Such steps are likely to be all the more useful when the mismatch between vaccination schedules in different parts of the world are taken into consideration.
In fact, in the final analysis, only global vaccination efforts timed to occur almost simultaneously, along with a strict temporary lockdowns, will be capable of reducing the worldwide spread of a resistant pandemic virus.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources