Coronavirus disease 2019 (COVID-19), caused by the novel coronavirus named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has caused a global pandemic of unprecedented proportions, overwhelming public health systems and economies across the world.
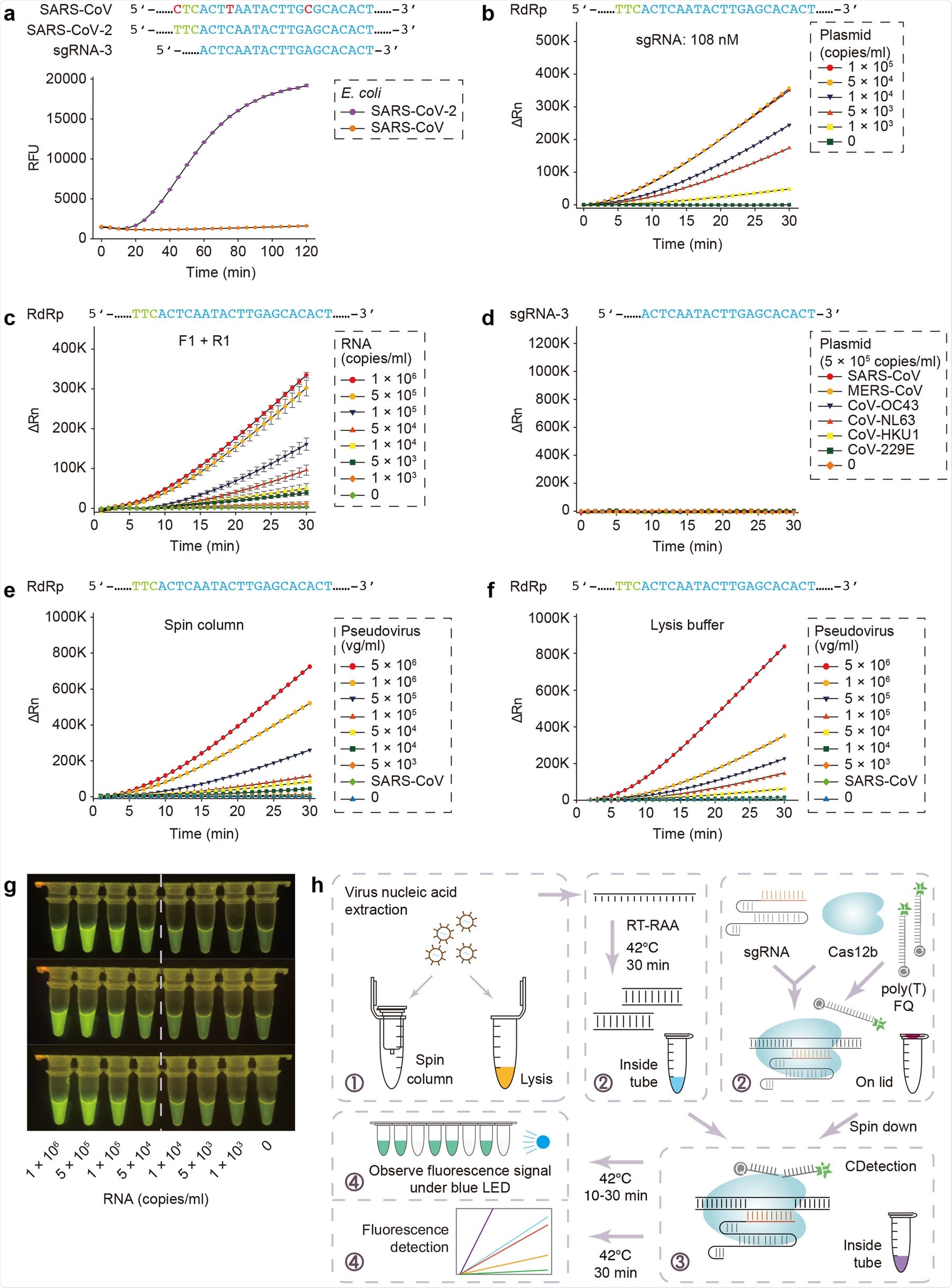
CASdetec used for SARS-CoV-2 detection. a Fluorescence kinetics of sgRNA-3 for RdRp detection. E. coli cells bearing Blunt-SARS-CoV-RdRp or Blunt-SARS-CoV-2-RdRp were pre-incubated at 95 °C for 10 min and used as templates for RAA and CDetection. PAM sequences are colored in green, protospacers are colored in blue, base pair mismatches are colored in red. Error bars indicate standard errors of the mean (s.e.m.), n = 3. RFU, relative fluorescence units. b Fluorescence kinetics of RdRp detection using 108 nM sgRNA-3. Plasmid bearing SARS-CoV-2-RdRp was serially diluted as shown in the legend. n =2. ΔRn, ΔFluorescence, which refers to the Rn value of an experimental reaction minus the Rn value of the baseline signal generated by ABI 7500. c Fluorescence kinetics of F1- and R1-based RdRp detection. SARS-CoV-2-RdRp RNA was serially diluted as shown in the legend. Error bars indicate (s.e.m.), n =3. d Evaluation of cross-reactivity. Plamids containing target RdRp region from six human epidemic coronaviruses were serially diluted as the shown in the legend. n =2. e Detection of SARS-CoV-2 pseudovirus. Virus genome was extracted using the virus RNA extraction kit (spin column). SARS-CoV were diluted to 5 × 105 copies/mL. n =2. f Detection of SARS-CoV-2 pseudovirus. Virus was treated by direct lysis. SARS-CoV was diluted to 5 × 105 copies/mL. n = 2. g CASdetec results could be directly observed under blue LED. 3 replicates of products from Fig. 1c were imaged upon blue LED illumination. h Schematics showing the workflow of CASdetec. Virus genome was extracted by kit or direct lysis. Target sequences were pre-amplified by isothermal amplification, followed was CDetection. Fluorescence signals were obtained either from fluorescence reader or direct observation under blue light.
New CRISPR-assisted nucleic-acid detection platform - CASdetec
To add to existing CRISPR-based nucleic acid detection protocols, a team of researchers recently established a new SARS-CoV-2 detection protocol based on their previously reported platform - CDetection (Cas12b-mediated DNA detection). They combined nucleic-acid amplification methods and sample treatment protocols with CDetection and established an integrated nucleic acid detection platform called CRISPR-assisted detection or CASdetec. CASdetec has a detection limit of 1×104 copies/mL for SARS-CoV-2 pseudovirus, without any observed cross-reactivity.
According to the available SARS-CoV-2 genome sequence, they designed 7 sgRNAs around the RdRp locus, as per World Health Organization (WHO) recommendation. Due to the high similarity of SARS-Cov-2 to SARS-CoV, they conducted initial experiments on the plasmids of both viruses. Fluorescence kinetics studies show that sgRNA-3 was distinct in that it could distinguish between the 2 coronaviruses and also produced the most distinct fluorescence signal.
The chosen sgRNA and primers can be used for most of the reported SARS-CoV-2 genomes
Since CRISPR cannot detect DNA at <1–10 nM of amplification product in the reaction mix, increasing molecular collisions between the target and CRISPR is crucial to improve the test's sensitivity. The researchers found that increasing the concentration of sgRNA by 3 folds enhances the fluorescence signal as well as the signal-to-background ratio. Moreover, it also boosts the rate of reaction. They designed and screened RAA primers to match their previously optimized sgRNA-3. The screenings showed that by using the best primer pairs with sgRNA-3, detection of nucleic acid is possible in SARS-CoV-2 RNA samples with 5 × 103 copies/mL.
The researchers also analyzed all 1792 SARS-CoV-2 sequences on GISAID until 26 March 2020 and found that 1673 of those sequences match their chosen primers and sgRNA. Only 3 of them has 1 mismatch to the forward primer, and only 2 of them had 1 mismatch to the reverse primer, which suggests that the sgRNA and primers they have chosen can be used for most of the reported SARS-CoV-2 genomes.
In order to validate the specificity of this new method for detection of SARS-CoV-2 nucleic acid, they tested the protocols against 6 coronaviruses - SARS-CoV, MERS-CoV, CoV-HKU1, CoV-229E, CoV-OC43, and CoV-NL63 - that cause respiratory illnesses. The results show no cross-reactivity with other endemic coronavirus and suggested that their sgRNA and primers had high sensitivity and specificity.
CASdetec assay design and optimization process could offer guidance for future CRISPR-based nucleic acid detection
To summarize, the authors established a new platform for nucleic acid detection based on CRISPR – the CASdetec. The platform has many procedures, including nucleic acid amplification, virus handling, and CRISPR-based detection. CASdetec is capable of detecting pseudovirus samples with >1 × 104 copies/mL without any cross-reactivity to other coronaviruses.
Additionally, the researchers also optimized the workflow to perform both reactions in a single tube without opening the lid. This will help prevent aerosol contamination and decrease the rate of false-positive test results.
According to the authors, in order to ensure the accuracy of CASdetec, users need to be careful to avoid nucleic acid aerosol contamination. They can do the following to avoid contamination: (a) perform sample treatment, reagent preparation, amplification, and product analysis in different rooms; and (b) carefully handle nucleic acid samples and reagents to avoid contamination through touch.
The researchers recently presented their assay design and optimization process in a Correspondence in the journal Nature. They believe that this process could offer guidance for future nucleic acid detection assay development and optimization based on CRISPR.
The team concludes:
We have established a CASdetec platform, which consists of procedures including virus handling, nucleic acid amplification, and CRISPR-based detection."