A new study suggests that the rollout of vaccines against the coronavirus disease 2019 (COVID-19) pandemic may be significant not only in preventing infections by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) but other endemic and pandemic-potential coronaviruses (CoVs) as well.
SARS-CoV-2 vaccines elicit cross-reactive CoV antibodies in humans
The use of SARS-CoV-2 vaccines in humans was associated with marked increases in antibodies against this virus, of course, but also of cross-reactive antibodies against SARS-CoV-1 and the common cold coronavirus OC43 in people with or without a history of infection with SARS-CoV-2.
COVID-19 patients also developed antibodies that recognized all three viruses.
Mice studies
Mouse studies then showed that vaccination against SARS-CoV-2 protected the animals against SARS-CoV-2 and SARS-CoV-1. Various vaccine platforms were tested, including adenovirus vector-based, mRNA-based, inactivated virus-based, spike-based or those based only on the receptor-binding domain (RBD), with the same results.
An experimental SARS-CoV-1 spike vaccine has been shown earlier to protect against SARS-CoV-1 infection in mice. Sera from these immunized mice showed cross-reactive partial inhibition of SARS-CoV-2 pseudovirus entry into cells in cell cultures.
Mice vaccinated with a SARS-CoV-1 vector vaccine were protected 280-fold better when challenged subsequently with SARS-CoV-2.
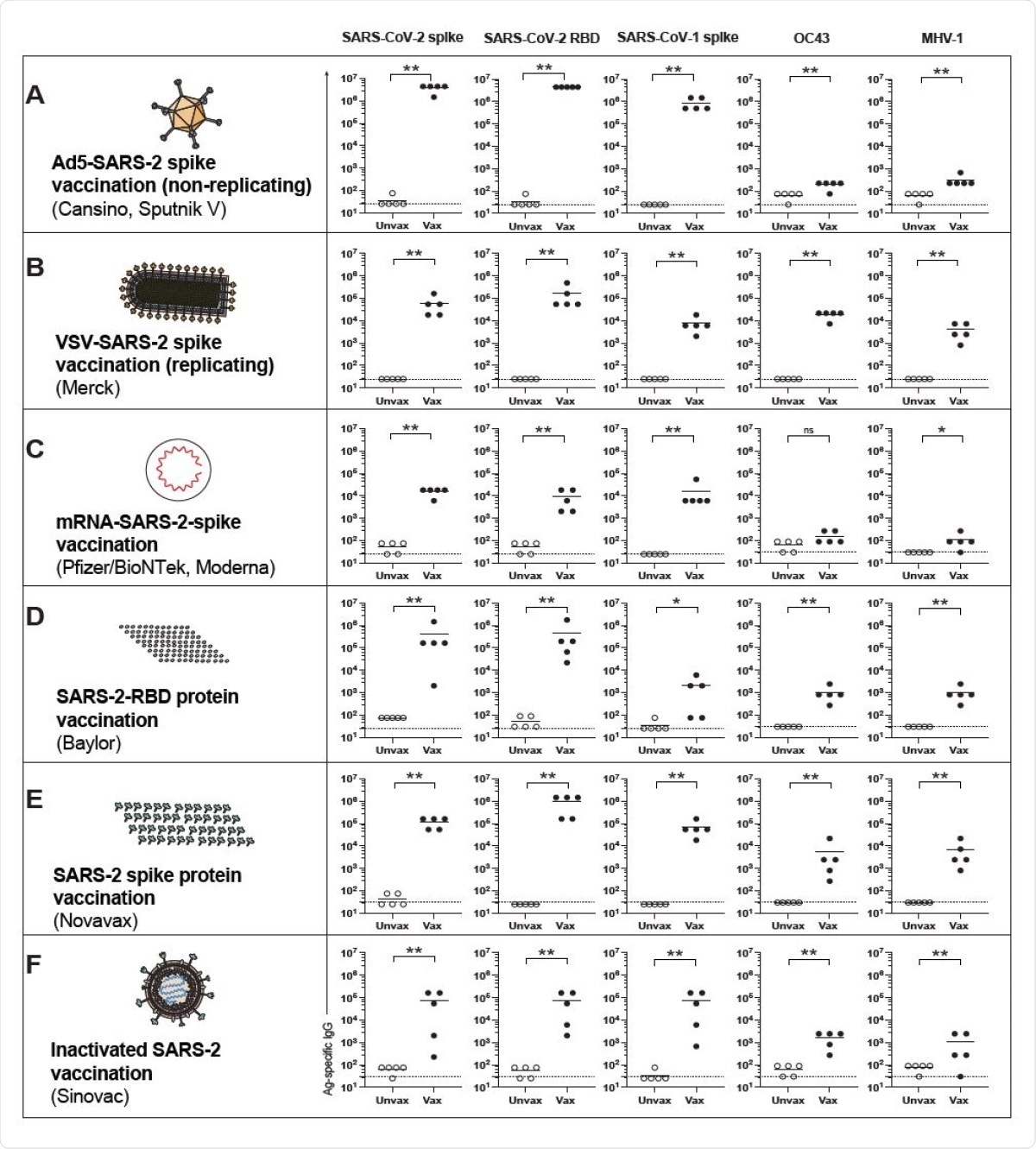
Cross-reactive antibody responses following SARS-CoV-2 vaccination in mice. (A) Antibody responses after Ad5-SARS-CoV-2 spike vaccination. (B) Antibody responses after VSV-SARS-CoV-2 spike vaccination. (C) Antibody responses after mRNA-SARS-CoV-2 spike vaccination. (D) Antibody responses after SARS-CoV-2 RBD vaccination. (E) Antibody responses after SARS-CoV-2 “whole” spike vaccination. (F) Antibody responses after inactivated SARS-CoV-2 vaccination. Mice were primed intramuscularly and boosted after 3 weeks (see Materials and Methods for vaccine dosing information). Antibody responses were evaluated by ELISA at week 2 post-boost. Experiments were done using wild type C57BL/6 mice, except for VSV-SARSCoV- 2 spike vaccination, which used k18-hACE2 (C57BL/6) mice. In each panel we indicate in parenthesis examples of clinically approved and experimental SARS-CoV-2 vaccines that are based on the same vaccine modality. Dashed lines represent limit of detection. Data are from 1 representative experiment with n=5/group; experiments were performed 2-3 times with similar results. *, P <0.05, **, P <0.01, ns, P > 0.05 by Mann Whitney U Test.
Gene identity mediates cross-reactivity
In mice, the SARS-CoV-1 vaccine-induced specific CD8+ T cells to SARS-CoV-2. Protein subunit and inactivated virus vaccines do not produce the viral protein within the host cells, and therefore do not elicit this response, unlike the other vaccine platforms.
Cross-reactive responses are mediated by the identity of viral genes between different viruses. Since SARS-CoV-1 and SARS-CoV-2 spike proteins share 76% antigenic identity, vaccination with the former induces strongly protective antibodies to the latter.
The study identified two highly conserved antibody binding sites on the spike protein, which may be involved in this cross-reactive T cell response.
Conversely, the 37% identity of the SARS-CoV-2 spike with the OC43 spike meant that SARS-CoV-2 spike-based vaccination failed to protect against the latter virus. However, the use of a nucleocapsid-based SARS-CoV-2 vaccine did provide partial protection, despite only 38% identity.
Again, spike-based vaccines were more effective at eliciting cross-reactive antibodies compared to those based on the RBD, probably because there are a greater number of conserved epitopes in the former, inducing a broader CoV response.
Cross-reactivity following infections
Infections with any CoV elicited antibodies protective against later infections with other CoVs as well. In particular, mouse infections with endemic human CoVs were associated with enhanced (320-fold) protection against other mice CoVs. Conversely, infection with one mouse CoV in mice led to sterilizing immunity against other mice CoVs.
T cells crucial in cross-reactive immunity
Both T cell-mediated and humoral immunity were associated with this cross-protective response to vaccination. Using a dendritic cell (DC)-based vaccine designed to avert antibody responses, but including antigenic peptides from the SARS-CoV-2 spike, envelope, membrane and nucleocapsid proteins, the researchers obtained CD8+ T cells.
Strikingly, this vaccine protected against challenge by a mouse CoV. Immune sera elicited by a SARS-CoV-1 spike vaccine protected naïve mice against mouse CoV infection, despite the fact that both spike antigens have only 30% identity.
What are the implications?
The researchers write:
These data demonstrate that coronavirus infections can confer partial or full protection against future infections with other coronaviruses. We also observed a pattern in which the degree of heterologous protection appeared to be proportional to the genetic similarity between the initial coronavirus and the subsequent coronavirus infection.”
An important implication of these findings is that antibodies elicited by any of the variants are likely to be protective against infection by another strain because the different SARS-CoV-2 variants have 99% identical spike antigens.
Another observation is the increased efficacy of infection in inducing cross-reactive antibodies compared to vaccination. The reason is that viral infections induce antibodies to a number of antigens, most of which are conserved between CoVs. On the other hand, most coronavirus vaccines use the spike antigen, which is the least conserved among CoV proteins.
This may suggest that additional viral antigens need to be added in designing next-generation vaccines to enhance the breadth of CoV coverage and thus provide a pancoronavirus vaccine.
The duration of immunity remains in question. This will depend, in part, on whether these broadly protective antibodies are produced at plasmablast levels or by plasma cells. The former is a short-lived cell type that produces highly specific cross-reactive antibodies during the acute phase of the infection, while plasma cells produce a durable response.
The SARS-CoV-2 nucleocapsid protein is composed of several sequences, some of which are highly conserved. In fact, one is almost exactly the same in human CoVs like OC43, bat CoVs and mouse CoVs.
Such long conserved sequences are perhaps implicated in eliciting cross-reactive T cells against CoVs in general. If so, the nucleocapsid may be the best tool for a universal CoV vaccine.
This study is “the first definitive demonstration of heterologous immune protection following coronavirus vaccination or infections.” By showing that prior CoV infection and CoV-based vaccines elicit cross-reactive protection against other CoVs, the researchers suggest an approach to vaccine design covering a broader range of pathogens.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.