A recent study conducted by an international team of scientists has revealed that the Oxford-AstraZeneca coronavirus disease 2019 (COVID-19) vaccine, AZD1222, is capable of inducing strong spike-specific CD4+ T cell helper type 1 (Th1) and CD8+ T-cell responses in vaccinated individuals. A detailed description of AZD1222-induced cellular immunity is currently available on the medRxiv* preprint server.
The Oxford-AstraZeneca-developed AZD1222 is a replication-deficient adenovirus vector-based COVID-19 vaccine, which has shown significant efficacy in preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and symptomatic COVID-19 in both clinical trials and real-world pandemic situation.
Besides inducing robust anti-spike antibody responses, AZD1222 has been found to trigger strong spike-specific effector T cell responses in adults at day 14 post-vaccination.
Studies characterizing AZD1222 efficacy have revealed that T cell responses can be detected as early as day eight and maintained through day 56 post-vaccination. However, AZD1222 has not yet been characterized extensively in terms of T cell cytokine responses.
In the current study, the scientists have characterized functional CD4+ and CD8+ T-cell responses in healthy adults after administering 1st and 2nd doses of the AZD1222 vaccine. Moreover, they have described the breadth and depth of spike-specific T cell responses induced by AZD1222.
A total of 280 participants who received two doses of AZD1222 vaccine were analyzed for spike-specific cytokine secretion and T cell receptor sequencing.
Important observations
To determine spike-specific T cell response in vaccinated individuals, the scientists experimentally stimulated cytokine production in participant-derived peripheral blood mononuclear cells with peptide pools that cover the entire spike sequence of SARS-CoV-2. The findings revealed that compared to the baseline (day 0), the total spike-specific CD4+ T cell helper type 1 (Th1) response increased significantly at days 28 and 56 post-vaccination.
The cytokines produced by Th1 cells primarily included TNF, followed by Interleukin-2 (IL-2), and Interferon-gamma (IFNγ). However, no Th2 response was observed in AZD1222-vaccinated individuals.
In contrast to CD4+ Th1 responses, a significantly lower frequency of spike-specific CD8+ T cells was detected in vaccinated individuals at days 28 and 56 post-vaccination.
A significant difference in cytokine production pattern was also observed between CD4+ and CD8+ T cells, with CD8+ T cells primarily producing IFNγ, followed by TNF and IL-2.
Together, these observations suggest that AZD1222 induces robust Th1 responses and significant expansion of CD8+ T cell responses.
Although a significantly increased CD4+ T cell response was observed across all age groups tested (18–55, 56–69, and ≥70 years), the kinetics of Th1 response was found to vary with age. Specifically, the findings revealed that AZD1222 induces polyfunctional Th1 response and CD8+ T cell response with similar frequency and functionality at day 56 post-vaccination across all age groups tested.
To determine the diversity and specificity of spike-specific T cell responses, the scientists performed sequencing of T cell receptor beta chain on peripheral blood mononuclear cells derived from AZD1222-vaccinated individuals at day 0 and day 28 post second dose. The findings revealed that after receiving the 2nd vaccine dose at 4 weeks or 12 weeks interval, the participants had significantly increased fractions of spike-specific total T cell receptors (depth of the response) and unique T cell receptors (breadth of the response).
Specifically, a significantly increased spike-specific T cell receptor breadth was observed at day 28 post second dose across all age groups tested. Similarly, a significantly increased spike-specific T cell receptor depth was observed at day 28 post second dose.
The mapping of each unique spike-specific T cell receptor sequence to a specific spike protein region revealed that spike-specific CD4+ T cell responses spanned the entire spike protein, with dominant responses observed in the N-terminal region 160–218 the C-terminal region 743–854.
In summary, these results suggest AZD1222 vaccination results in a significant expansion of spike-specific T cells whose receptor sequences map to multiple epitopes in the spike protein.
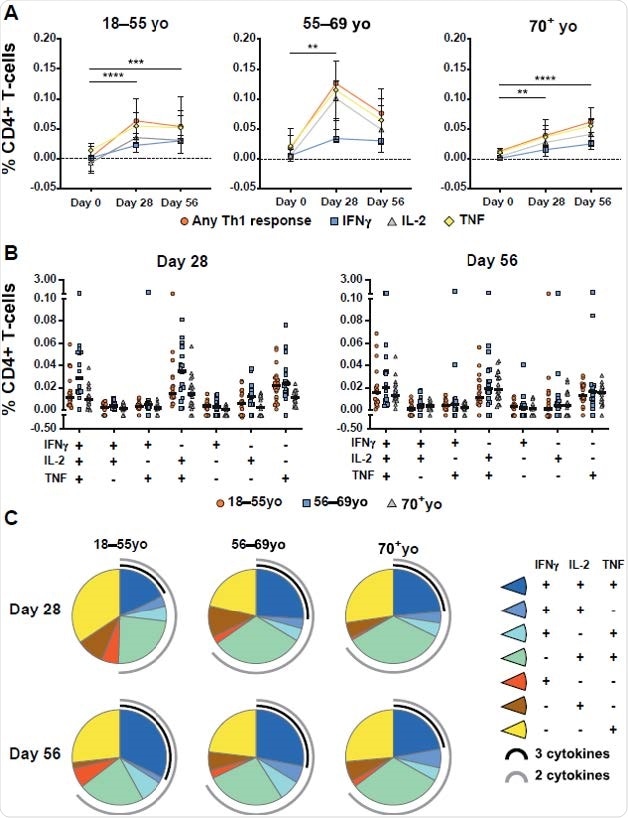
Age-specific CD4+ T-cell responses to AZD1222 vaccination. (A) Median frequencies with interquartile ranges of CD4+ T cells from participants within each age cohort producing IFNγ, IL-2, TNF, or any combination of these cytokines at the indicated timepoints following stimulation with SARS-CoV-2 spike peptide pools. Significant differences between timepoints within each vaccine group were determined by Kruskal-Wallis tests with Dunn’s test to correct for multiple comparisons. **p<0.01, ***p<0.001, ****p<0.0001. (B) Frequencies of antigen-stimulated CD4+ T cells producing each combination of IFNγ, IL-2, TNF cytokines at Day 28 (left) or Day 56 (right) post-vaccination. Individual participant responses are shown with median represented by the horizontal line. (C) Pie graphs indicating the total proportion of spike-specific Th1 cytokine production averaged for all participants within the indicated age groups at Day 28 and Day 56 post vaccination. Proportion of multicytokine responses are represented by the black (three cytokines) and gray (two cytokines) arcs.
Study significance
The study reveals that the AZD1222 vaccine could provide long-lasting protection against COVID-19 by significantly inducing polyfunctional Th1-dominated T cell responses to SARS-CoV-2 spike protein. Moreover, the vaccine causes a significant expansion of spike-specific CD4+ and CD8+ T cells with unique T cell receptor sequences that map to multiple spike epitopes.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources