The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which first emerged in Wuhan, China, in late December 2019, has quickly become the most urgent issue facing scientists worldwide. It has been reported that the respiratory infection causes cardiac dysfunction in 25-55% patients, including hospitalized patients and non-critical patients.
SARS-CoV-2 is responsible for triggering respiratory illness leading to symptoms such as difficulty in breathing and chronic pulmonary and cardiovascular hypoxia. Research has shown that under low oxygen conditions associated with COVID-19, the hypoxia-inducible factor 1 alpha (HIF1α), known to be the master regulator of hypoxia, gets stabilized and protects against SARS-CoV-2 infection.
“This study establishes a direct link of cardiac cellular responses to hypoxic stress with matching functional and histological data, serving as one of the first studies to bridge previous stand-alone clinical data and cellular data,” says the team.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
How does HIF1α protect COVID-19?
As mentioned above, under low oxygen conditions, HIF1α does not undergo oxygen-dependent proteolysis and accumulates in the nucleus. This accumulation results in dimerization with HIF1β, which then binds to the hypoxia-response elements (HREs).
“HIF1α stabilization leads to an avalanche of transcriptional activities involving angiogenesis, proliferation, homeostasis, inflammation, and metabolic switch”, says the team.
A recent study has shown that stabilization of HIF1α leads to a decrease in ACE2 receptors, which are the entry point of the SARS-CoV-2 virus. A different study also suggested that a decrease in SARS-CoV-2 pathogenicity at high altitudes was also due to a decrease in ACE2 receptors in response to hypoxic environments.
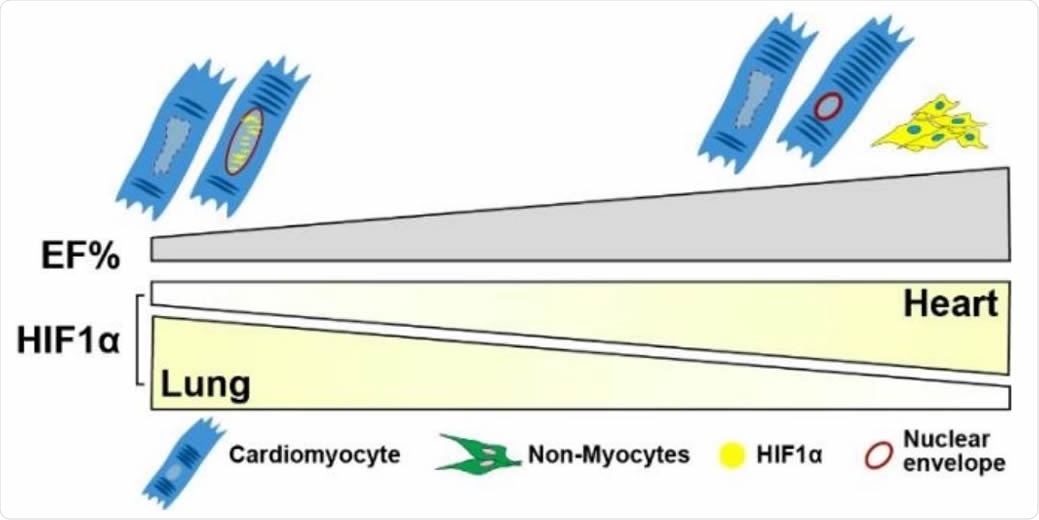
Schematic of HIF1α levels versus EF% in lung and heart. HIF1α expression is decreased in the lungs of COVID-19 patients with preserved cardiac EF but inversely, noted to be increased in the hearts of the same patients. In the preserved EF group (EF 58-63%), more HIF1α cells were detected predominantly in NCM cytoplasm (yellow shade). In low EF group (EF 33-43%), overall HIF1α+ cell quantity is lower whereas most HIF1α expression is detected in CM nuclei (yellow speckles). In cardiomyocytes, nuclear HIF1α appears to be protective by preserving nuclear Lamin and morphology. Myocyte nuclei that lack HIF1α expression appear to be enlarged and leaky with undetectable nuclear envelopes.
What did the study involve?
This study involved two groups of COVID-19 patients, those with preserved cardiac function having Ejection Fraction (EF) greater than 50 percent and those with moderate to severe cardiac dysfunction having EF less than 45 percent.
The echocardiography data, which involved both systolic and diastolic function, was examined along with post-mortem samples. Additionally, the heart samples were immunostained with primary and secondary antibodies.
The study of apoptosis was carried out by the hep of TUNEL assay, which used in situ cell death detection kit. Quantitative RT-PCR was carried out of the post-mortem lungs to detect the presence of RNA. Finally, Transmission Electron Microscopy (TEM) was performed on a control heart sample that did not have COVID-19 history.
What did the study find?
The researchers found that in preserved hearts, the expression of HIF1α is mostly in non-myocytes that help protect endothelial cells.
“Endothelial cells adapt to hypoxia by activating HIF1α and orchestrating a number of genes involving cellular metabolism, anti-apoptosis, and proinflammatory response”, says the team.
The nuclear envelope in low EF heart is thinner than preserved EF heart which results in loss of function, sarcomeric damage, and myofibril abnormalities.
“These data suggest that cell-type dependent HIF1α expression is increased in hearts of COVID-19 patients with preserved cardiac function”, adds the team.
What did the authors conclude?
“Our data provide compelling evidence of the protective role played by HIF1α in hearts of patients affected by COVID-19”, says the team.
The protective role of HIF1α in hearts may not only help to predict cardiac involvement in COVID-19 patients but also help to understand mechanisms involved in other forms of viral cardiomyopathy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources