Scientists at Tel Aviv University in Israel have successfully printed an entire active and viable brain tumor for the first time, using a three-dimensional (or 3D) printer. The researchers recreated the flowing blood vessels and surrounding brain tissue.
The 3D model contains all components of the malignant tumor. It could be the basis for potentially replacing cell cultures and animal models as a powerful platform for drug target discovery, personalized therapy, and developing effective drugs.
Glioblastoma
Glioblastoma (GB) is the most common malignant brain tumor accounting for 47.7 percent of all cases. It is also the most aggressive type of brain tumor, affecting 3.21 people per 100,000 in the United States alone. The incidence has been on the rise in many countries.
Cancer is one of the leading causes of death across the globe. Approximately 30 to 40 percent of cancer patients are being treated with ineffective drugs. Thus, preclinical drug screening platforms aim to overcome this challenge. Unfortunately, most of the existing methods to identify druggable targets have limited efficacy. There is a need for a reliable and clinically relevant platform for high-throughput drug screening.
3D printing
The conventional preclinical drug development process relies on the in vitro evaluation of drug efficacy and toxicity in two-dimensional (2D) cell culture followed by animal studies. Over the years, 2D culture studies have been used in biomedical research and drug screening because it is cost-effective. However, the method struggles to predict the diverse effects of treatment in vivo.
The emergence of 3D models shows promise in overcoming previous cancer models' limitations and reducing the costs of preclinical drug evaluation. Previous models have been developed, but they lack the plethora of stromal cells and functional blood vessels, which are essential for disease development and progression, and evaluation of the response to treatment.
3D-bioprinted engineered tumor model
Promising novel technology in tissue regeneration is 3D-bioprinting, a technology for accurate 3D construction of complex tissues and organs. This technology allows the positioning of cells and biocompatible materials layer by layer. It also allows the use of a broad range of biomaterials at various viscosities and cell densities. The technology can better imitate the tumor microenvironment (TME), providing a glimpse of the complete physiological characteristics of the tumor, including blood vessels and multiscale architecture.
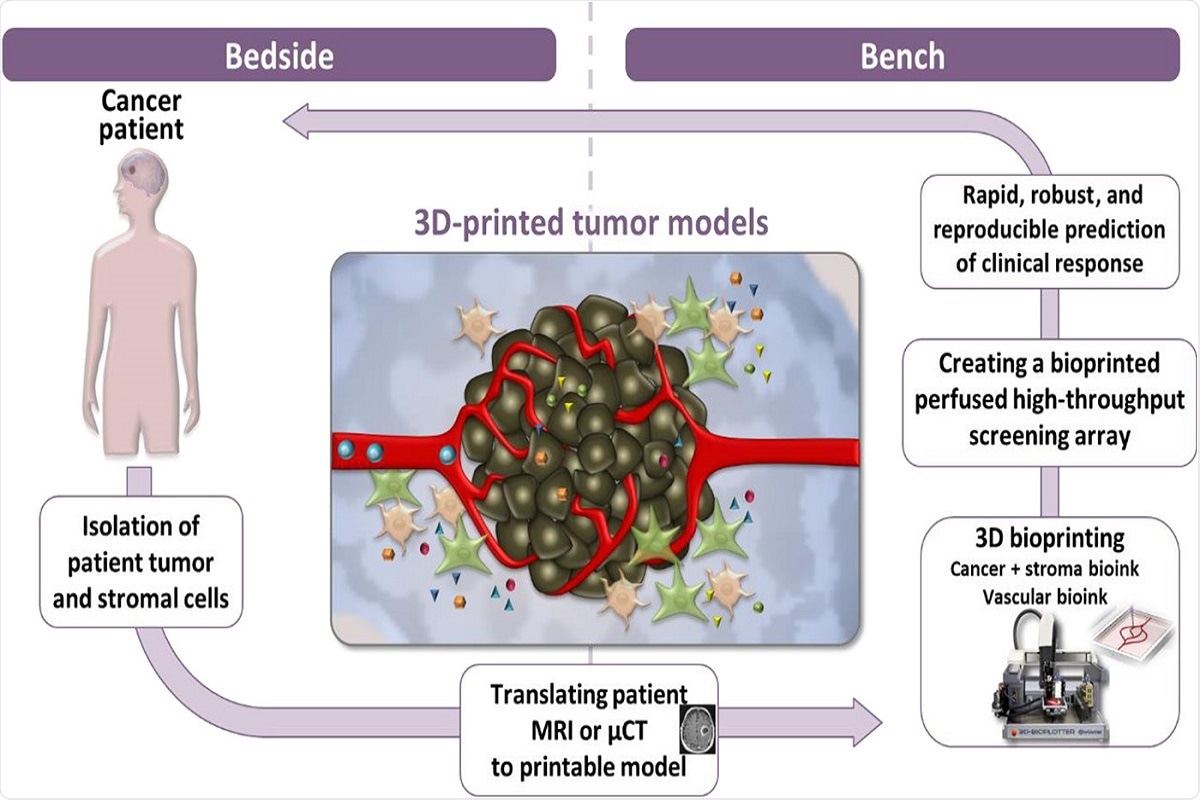
Fig. 7 Bridging the translational gap from bedside to bench and back. Schematic illustration of the methodological approach using a perfusable microengineered vascular 3D-bioprinted tumor model for drug screening and target discovery. MRI, magnetic resonance imaging; μ-CT, micro–computed tomography.
The team developed a 3D-bioprinted engineered tumor model based on two bio-inks, a tumor bio-ink and a vascular bio-ink. They focused on 3D-bioprinting of glioblastoma because the intratumoral heterogeneity and the TME are the significant drivers of GB cell resistance to therapy. Developing models that mimic the complex microenvironment of GB shows promise in facilitating the development of effective treatment options.
After successfully printing the 3D tumor, the researchers demonstrated that unlike cancer cells growing on Petri dishes, the 3D-bioprinted model could offer a strong and fast prediction of the most suitable treatment for a specific patient with the capability of being reproduced.
The team investigated the mechanical properties and biological functionality of the biocompatible fibrin 3D-bio-ink. The models resembled GB cellular heterogeneity, cell-cell interaction, and spatial tomography.
The study findings showed similar growth curves, drug response, and genetic signature of glioblastoma cells grown in the 3D-bio-ink platform.
We demonstrate here that our 3D-bio-ink can serve as an alternative to mouse models, as it can mimic key features of tumors grown in vivo..."
The 3D-bioprinted GB model could help provide effective treatments as a personalized therapy and is significant for managing aggressive tumors that have short-term survival.