In the wave of the current coronavirus disease 2019 (COVID-19) pandemic, scientists have frequently had to revise earlier assumptions and conclusions as more data has been released on this disease.
 Study: Protective Activity of mRNA Vaccines Against Ancestral and Variant SARS-Cov-2 Strains. Image Credit: KT Stock Photos / Shutterstock.com
Study: Protective Activity of mRNA Vaccines Against Ancestral and Variant SARS-Cov-2 Strains. Image Credit: KT Stock Photos / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The Moderna COVID-19 vaccine is one of several that have gained emergency use authorization (EUA) in several countries around the world. All of the current vaccines were originally designed against the Wuhan or ancestral strain of SARS-CoV-2.
The emergence of multiple SARS-CoV-2 variants challenged the initial vaccine-related dive in the rates of cases, hospitalizations, and deaths, as these new strains have resisted neutralization by the antibodies elicited by infection with the earlier strain or vaccination.
These SARS-CoV-2 variants include several that pose a threat to containing viral spread, as they are either more infective, have higher transmissibility, and/or escape neutralization by antibodies against the earlier variants. In particular, several SARS-CoV-2 variants have been deemed variants of concern (VOCs), some of which include B.1.1.7 (Alpha), B.1.351 (Beta), B.1.1.28 (Gamma), and B.1.617.2 (Delta), with mutations in the spike at several locations.
Substitutions at L452 and E484 are associated with a loss of susceptibility to antibody-mediated neutralization. The drop in efficacy against the Beta variant has been most pronounced with adenovirus vectored vaccines such as the Oxford AstraZeneca vaccine and protein nanoparticle-based vaccines.
Conversely, some researchers have reported that mRNA vaccines continue to show 75% efficacy against the Beta variant and even higher protection against severe COVID-19.
In the current study, two modifications of the Moderna vaccine were tested. The first is a lipid nanoparticle-encapsulated mRNA-1273 vaccine with the prefusion-stabilized spike antigen from the Wuhan variant. Comparatively, the second modified vaccine contained the prefusion-stabilized Beta spike variant (mRNA-1273.351).
Clinical studies showed 94% efficacy against symptomatic disease, with the first confirmed by protection against respiratory infection in non-human primates immunized with two doses of the vaccine, and subsequently challenged with the Beta variant.
About the study
In the current study, the researchers compare the efficacy of these two modified vaccines against the Beta VOC in a mouse model. The vaccines used for the testing included high and low doses of mRNA-1273, mRNA-1273.351, and mRNA-1273.211 (a 1:1 mixture of both the preceding vaccines). This mixture is currently being tested in human clinical trials.
The first phase consisted of testing two breeds of mice immunized with these two modified mRNA-1273 vaccines, during which the researchers evaluated the level of protection offered by the vaccines against different SARS-CoV-2 variants. The mice expressed either native mouse angiotensin-converting enzyme 2 (ACE2), which is the receptor used by SARS-CoV-2 to gain entry into the cell, or were engineered to express human ACE2.
This meant that the mice could be challenged by either mouse-adapted strains of SARS-CoV-2 with the N501Y mutation or human SARS-CoV-2 variants.
Study findings
The researchers found that all three formulations produced a robust immunoglobulin G (IgG) response against the spike proteins of the ancestral (Wuhan-1) and Alpha, Beta, and Delta strains. Furthermore, the antibody titers ranged from 600,000 to 1,500,000 at the higher dose of 5 micrograms (μg), with no significant difference between the vaccines.
At the lower dose of 0.25 μg, the titers were five-fold lower against all spike variants.
Reduced efficacy against Delta VOC
All vaccines elicited strong neutralizing responses to the spike variants, though the mRNA-1273.351 produced a two-fold higher geometric mean titer (GMT) against the Beta variant, and four-fold lower against the D614G variant. The neutralizing activity against the Delta variant was reduced four- to five-fold for all the vaccines at the higher dose, and by ten-fold at the lower dose.
Neutralizing antibody levels with the mRNA-1273.351 vaccine were lower for the D614G with or without the N501Y mutation. Comparatively, the antibody levels attained with the mRNA-1273.211 mixture showed no increase over those achieved with just one of the component vaccines.
The mRNA-1273 vaccine produced lower neutralization against the Beta variant than expected from earlier studies in both mice and humans.
An antigenic map showed that the Delta spike variant is the most distant from the others, partially explaining why it induces lower levels of neutralizing antibodies with any vaccine.
Disease prevention
When exposed to the viral variants WA1/2020 N501Y/D614G, B.1.1.7/E484K, or B.1.351, the vaccines protected the mice against weight loss. The viral load was lower in the nose, lungs, and spleen of the mice, thereby indicating that the vaccines prevented infection in the majority of cases.
Moderate infection with the B.1.1.7/E484K variant occurred in 5/8 and 3/8 of mice who received mRNA-1273 and mRNA-1273.211 vaccines, respectively. However, viral ribonucleic acid (RNA) material was either not found in the lungs or was present at greater than 100-fold reduced levels relative to control mice.
Complete protection was conferred by the mRNA-1273.351 vaccine at 5 μg. The lower dose was less or not protective against nasal infection with the Beta strain. Two doses were effective against lung infection with the WA1/2020 N501Y/D614G strain with all three vaccines. Breakthrough infection was observed after exposure to B.1.1.7/E484K and especially the B.1.351 strain.
Neutralizing antibody levels were negatively associated with viral RNA levels in the lungs. Furthermore, an antibody titer of 5,000 was mostly linked to protection against lung infection on the fourth day post-vaccination. The lowest level was required for prevention of WA1/2020 N501Y/D614G infection, at 2-7-fold lower. However, in most cases, the Beta variant was responsible for breakthrough infections at the lower vaccine dose.
High vaccine dose is protective
Again, decreased body weight was largely seen following exposure to the Beta strain. However, lung changes were absent, even at the lower dose.
“Immunization with even the low dose of the mRNA vaccines was sufficient to mitigate SARS-CoV-2-induced lung injury in immunocompetent mice challenged with some VOC.”
In mice expressing human ACE2 receptors, only the mRNA-1273 and mRNA-1273.351 were tested due to the small number of mice available. All antibody responses were somewhat reduced compared to the other mice, though still robust against the spike antigens.
Neutralizing activity was seen to be strong in all immunized mice at the higher dose, but lower with the mRNA-1273.351 against the D614G or D614G/N501Y strains, compared to the unmodified vaccine. The converse was true of viruses with the E484K mutations. Both were equally active against the Delta strain, though the titers were lower as compared to that which was observed with the other variants.
At lower doses, the neutralizing activity was ten-fold lower with both vaccines against all viral strains. Though similar trends were observed, B.1.1.7/E484K, B.1.351, and B.1.617.2 were neutralized less efficiently, especially after immunization with mRNA-1273. The antigenic map mirrored that observed with the mice expressing native ACE2 receptors.
Nasal, lung, and brain infection was prevented by high-dose vaccination with either vaccine, but protection was incomplete after the lower dose. Lung infection was observed with B.1.351, B.1.1.7/E484K, and B.1.617.2 strains in almost all animals, thus indicating an almost complete loss of protection with mRNA-1273.
With the mRNA-1273.351 vaccine, infection with B.1.351 was prevented. However, all viral variants led to infection in all sampled tissues for almost all immunized animals, except the brain which was involved in half the animals.
Neutralizing antibody titers were not necessarily correlated with clinical protection in the lung, though the loss of body weight showed a linear relationship.
“The best correlation was seen after B.1.617.2 challenge, with greater weight loss in mice immunized with the 0.25 μg vaccine dose and having lower serum neutralizing antibody titers.”
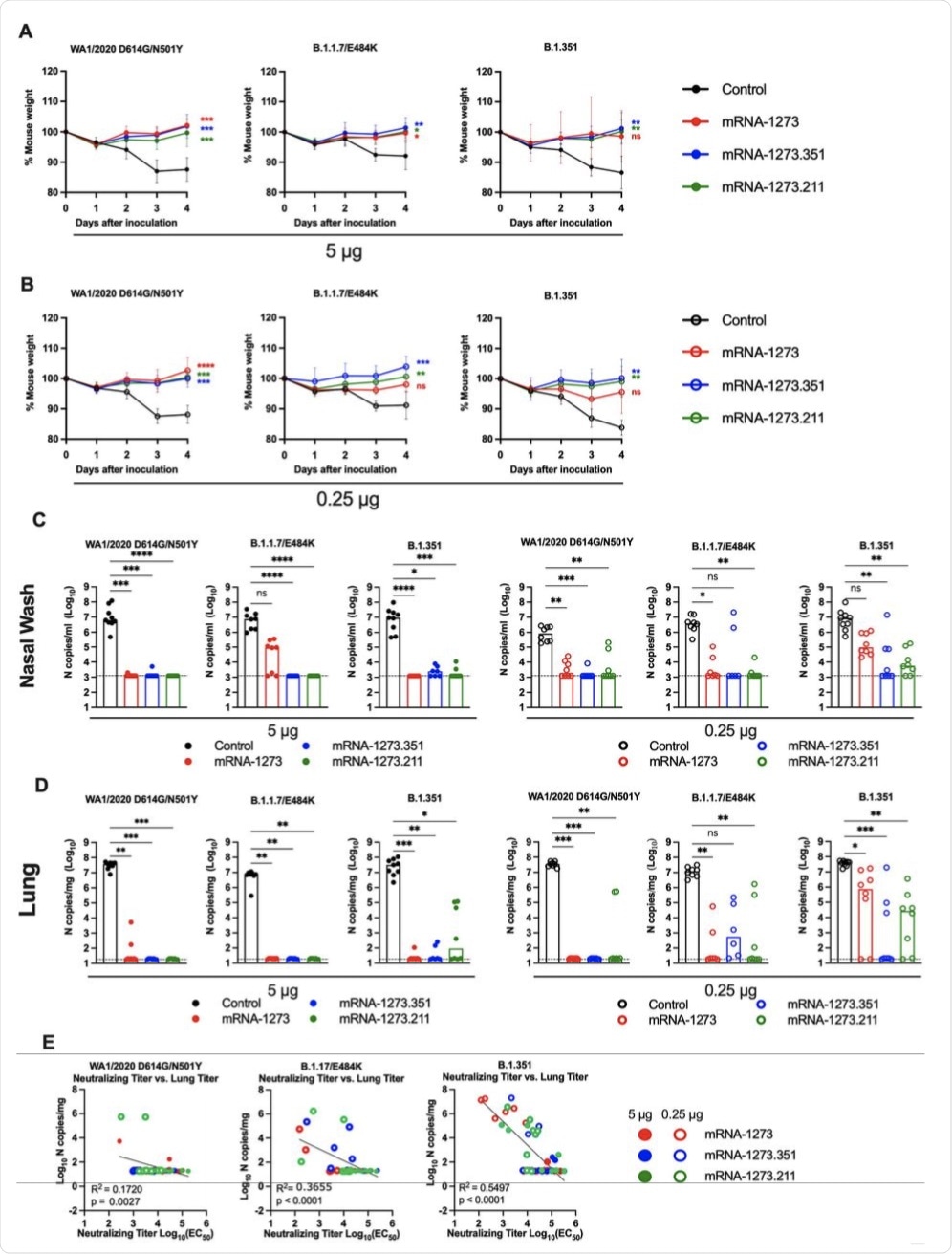 Seven to nine-week-old female 129S2 mice were immunized and boosted with 5 or 0.25 μg of mRNA vaccines (control (black symbols), mRNA-1273 (red symbols), mRNA-1273.351 (blue symbols), and mRNA-1273.211 [mixture, green symbols]) as described in Figure 1A. Three weeks after boosting, mice were challenged via intranasal inoculation with 105 focus-forming units (FFU) of WA1/2020 N501Y/D614G, B.1.1.7/E484K, or B.1.351. A-B. Body weight change over time. Data shown is the mean +/− SEM (n = 6-9, two independent experiments; one-way ANOVA of area under the curve from 2-4 dpi with Dunnett’s post-test, comparison to control immunized group: ns, not significant; P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; **** P < 0.0001). C-D. Viral burden at 4 dpi in the nasal washes (C) and lungs (D) as assessed by qRT-PCR of the N gene after challenge of immunized mice with the indicated mRNA vaccines (n = 6-8, two independent experiments, boxes illustrate median values, dotted line shows LOD; one-way Kruskal-Wallis ANOVA with Dunn’s post-test, comparison among all immunization groups: ns, not significant; P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; **** P < 0.0001). E. Correlation analyses comparing serum neutralizing antibody concentrations three weeks after boosting plotted against lung viral titer (4 dpi) in 129S2 mice after challenge with the indicated SARS-CoV-2 strain (Pearson’s correlation P and R2 values are indicated as insets; closed symbols 5 μg vaccine dose; open symbols, 0.25 μg vaccine dose).
Seven to nine-week-old female 129S2 mice were immunized and boosted with 5 or 0.25 μg of mRNA vaccines (control (black symbols), mRNA-1273 (red symbols), mRNA-1273.351 (blue symbols), and mRNA-1273.211 [mixture, green symbols]) as described in Figure 1A. Three weeks after boosting, mice were challenged via intranasal inoculation with 105 focus-forming units (FFU) of WA1/2020 N501Y/D614G, B.1.1.7/E484K, or B.1.351. A-B. Body weight change over time. Data shown is the mean +/− SEM (n = 6-9, two independent experiments; one-way ANOVA of area under the curve from 2-4 dpi with Dunnett’s post-test, comparison to control immunized group: ns, not significant; P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; **** P < 0.0001). C-D. Viral burden at 4 dpi in the nasal washes (C) and lungs (D) as assessed by qRT-PCR of the N gene after challenge of immunized mice with the indicated mRNA vaccines (n = 6-8, two independent experiments, boxes illustrate median values, dotted line shows LOD; one-way Kruskal-Wallis ANOVA with Dunn’s post-test, comparison among all immunization groups: ns, not significant; P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; **** P < 0.0001). E. Correlation analyses comparing serum neutralizing antibody concentrations three weeks after boosting plotted against lung viral titer (4 dpi) in 129S2 mice after challenge with the indicated SARS-CoV-2 strain (Pearson’s correlation P and R2 values are indicated as insets; closed symbols 5 μg vaccine dose; open symbols, 0.25 μg vaccine dose).
Notably, at both doses, the vaccinations successfully reduced intense inflammation in the lungs. Lung changes were prevented with all variants following mRNA-1273 vaccination, though incompletely following exposure to the B.1.351 variant.
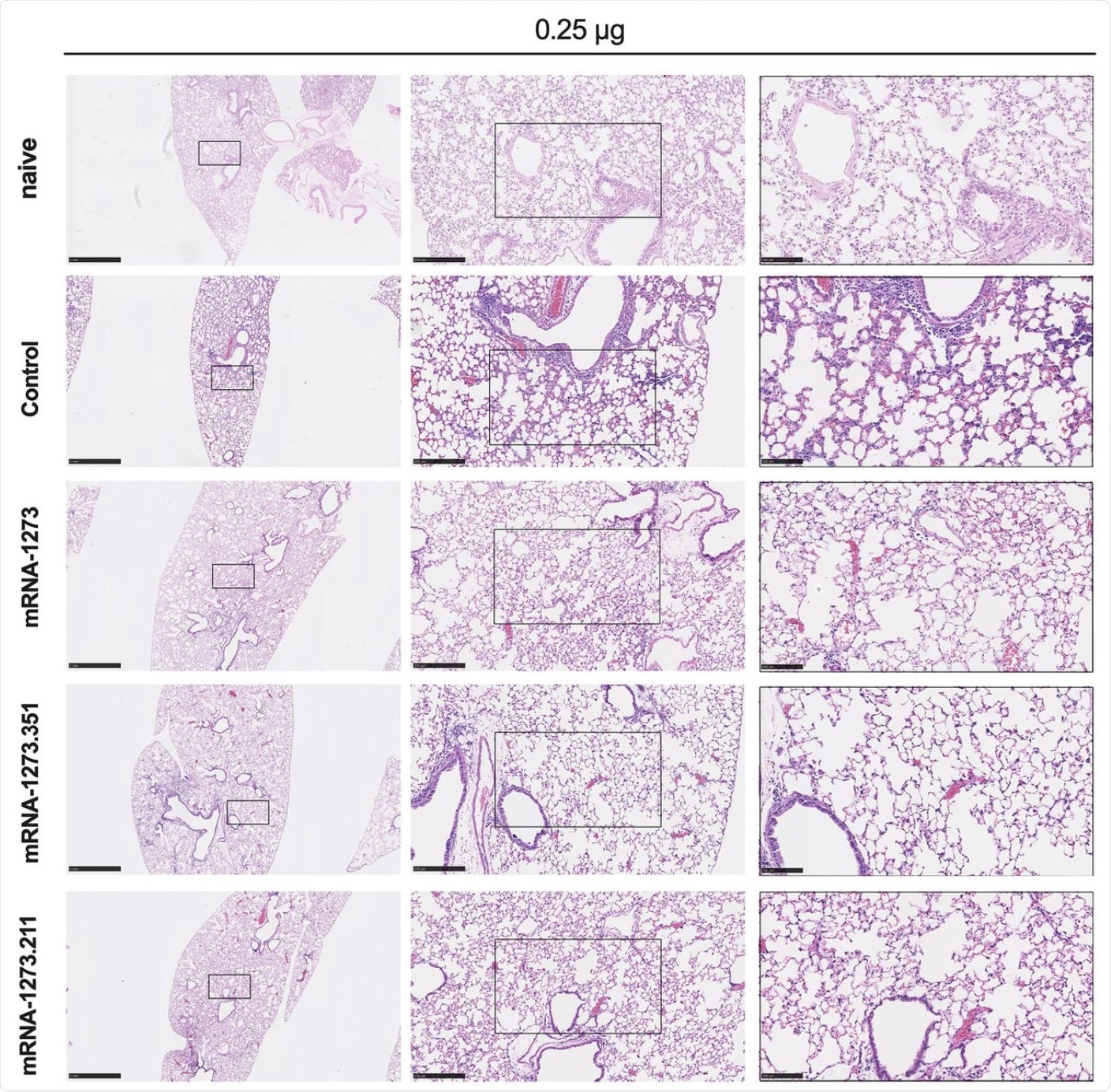 Seven to nine-week-old female 129S2 mice were immunized, boosted with 0.25 μg of mRNA vaccines (control, mRNA-1273, mRNA-1273.351, or mRNA-1273.211), and challenged with B.1.351 as described in Figure 2. Hematoxylin and eosin staining of lung sections harvested at 4 dpi or from a mock-infected animal. Images show low- (top; scale bars, 1 mm) and high-power (bottom; scale bars, 50 μm). Representative images from n = 2 per group.
Seven to nine-week-old female 129S2 mice were immunized, boosted with 0.25 μg of mRNA vaccines (control, mRNA-1273, mRNA-1273.351, or mRNA-1273.211), and challenged with B.1.351 as described in Figure 2. Hematoxylin and eosin staining of lung sections harvested at 4 dpi or from a mock-infected animal. Images show low- (top; scale bars, 1 mm) and high-power (bottom; scale bars, 50 μm). Representative images from n = 2 per group.
Conversely, with mRNA-1273.351, all variants were protected except in some mice challenged with WA1/2020 N501Y/D614G. The Delta variant did cause pneumonia after low-dose vaccination, thus indicating only partial protection.
Implications
The current study demonstrated that while both high-dose vaccine formulations showed robust antibody-mediated and T-cell immune protection against weight loss and pneumonia after challenge with all viral variants, the protection was incomplete with low-dose vaccines. This was particularly true following exposure to the Delta variant, where pneumonia was still detected following administration of low-dose formulations.
In mice expressing the human ACE2 receptor, the mRNA-1273 vaccine failed to induce strong responses against B.1.1.7/E484K and B.1.351, which also has the E484K mutation. The mRNA-1273.351 vaccine elicited higher neutralizing antibody titers against these variants, except for the WA1/2020 D614G, which induced a lower neutralizing response.
Notably, both vaccines were less protective against the Delta variant in both mouse models.
The 10- to 20-fold reduced peak neutralization titers and T-cell responses induced by lower vaccine doses relative to the higher doses were similar to the loss of 90% of neutralizing immunity three months after vaccination or natural infection in humans. Again, this difference was most obvious with the Delta variant.
This correlates with Delta-related breakthrough infections reported in regions with high vaccine coverage.
“Although studies in humans are required, boosting with historical or variant (e.g., mRNA encoding B.1.617.2 spike genes) vaccines might be required to prevent breakthrough events as vaccine-induced immunity wanes.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Ying, B., Whitener, B., VanBlargan, L. A., et al. (2021). Protective Activity of mRNA Vaccines Against Ancestral and Variant SARS-Cov-2 Strains. bioRxiv. doi:10.1101/2021.08.25.457693. https://www.biorxiv.org/content/10.1101/2021.08.25.457693v1.
- Peer reviewed and published scientific report.
Ying, Baoling, Bradley Whitener, Laura A. VanBlargan, Ahmed O. Hassan, Swathi Shrihari, Chieh-Yu Liang, Courtney E. Karl, et al. 2022. “Protective Activity of MRNA Vaccines against Ancestral and Variant SARS-CoV-2 Strains.” Science Translational Medicine 14 (630). https://doi.org/10.1126/scitranslmed.abm3302. https://www.science.org/doi/10.1126/scitranslmed.abm3302.