The worldwide outbreak of the coronavirus disease 2019 (COVID-19) pandemic, which was brought about by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused severe threats to the international health and economy.
Although several vaccines have been developed against COVID-19, the availability and accessibility of these vaccines are limited. Furthermore, there has been an increase in the incidence of immune escape, even after complete vaccination by several of these vaccines.
Currently, there are no specific antiviral drugs against SARS-CoV-2. Therefore, research into the development of anti-SARS-CoV-2 therapeutics remains essential.
The entry of SARS-CoV-2 into the host cell is governed by the viral structural spike (S) proteins, which form a crown-like structure on the surface of the virion. Interaction between the viral S protein and the host protein angiotensin-converting enzyme 2 (ACE2) receptor is essential for infection.
ACE2 has been found to be upregulated in patients suffering from severe COVID-19. This observation suggests that blockage of the S protein and the ACE2 can be considered as a target for the designing of drugs.
Extracellular vesicles (EVs), which are also referred to as natural “Trojan horses” for drug delivery and therapies, are cell-derived vesicles that have an average diameter of 100 nanometers (nm). Since EVs have shown great promise in serving as carriers of biomacromolecules, several pharmaceutical companies have invested in the research and development of therapeutic EVs.
A new study aimed to develop EVs with genetically engineered embedded ACE2 (termed EVs-ACE2) that serve as antagonists against the viral S protein. Taken together, these EVs were found to help inhibit the entry of the virus into the host cells, as well as neutralize the S-pseudovirus.
About the study
In the current study, the researchers discuss the preparation and characterization of EVs-ACE2, along with the production and characterization of SARS-CoV-2 S-pseudovirus. Investigation of inhibition of viral attachment and viral infection by EVs-ACE2 took place in vitro.
The in vivo inhibition test took place in 6- to 8-week old female mice. Free access to food and water was provided to the mice throughout the course of the experiment.
The mice were divided into three groups, which included the blank control, the negative control that received EVs-Control, and the experimental group that received EVs-ACE2. The nasal mucosa tissue was collected from the mice, which were then subjected to Western Blot analysis to determine the presence of ACE2 protein expression.
In vivo, preliminary safety studies were carried out, where EVs-ACE2 or EVs-Control were administered into the nasal cavity of the mouse, and blood from the orbital vein was collected three days later. The serum obtained from the blood was then analyzed with the help of an automated hematology analyzer for blood chemistry tests. Histopathological examination of different mice organs was also carried out using the hematoxylin-eosin staining.
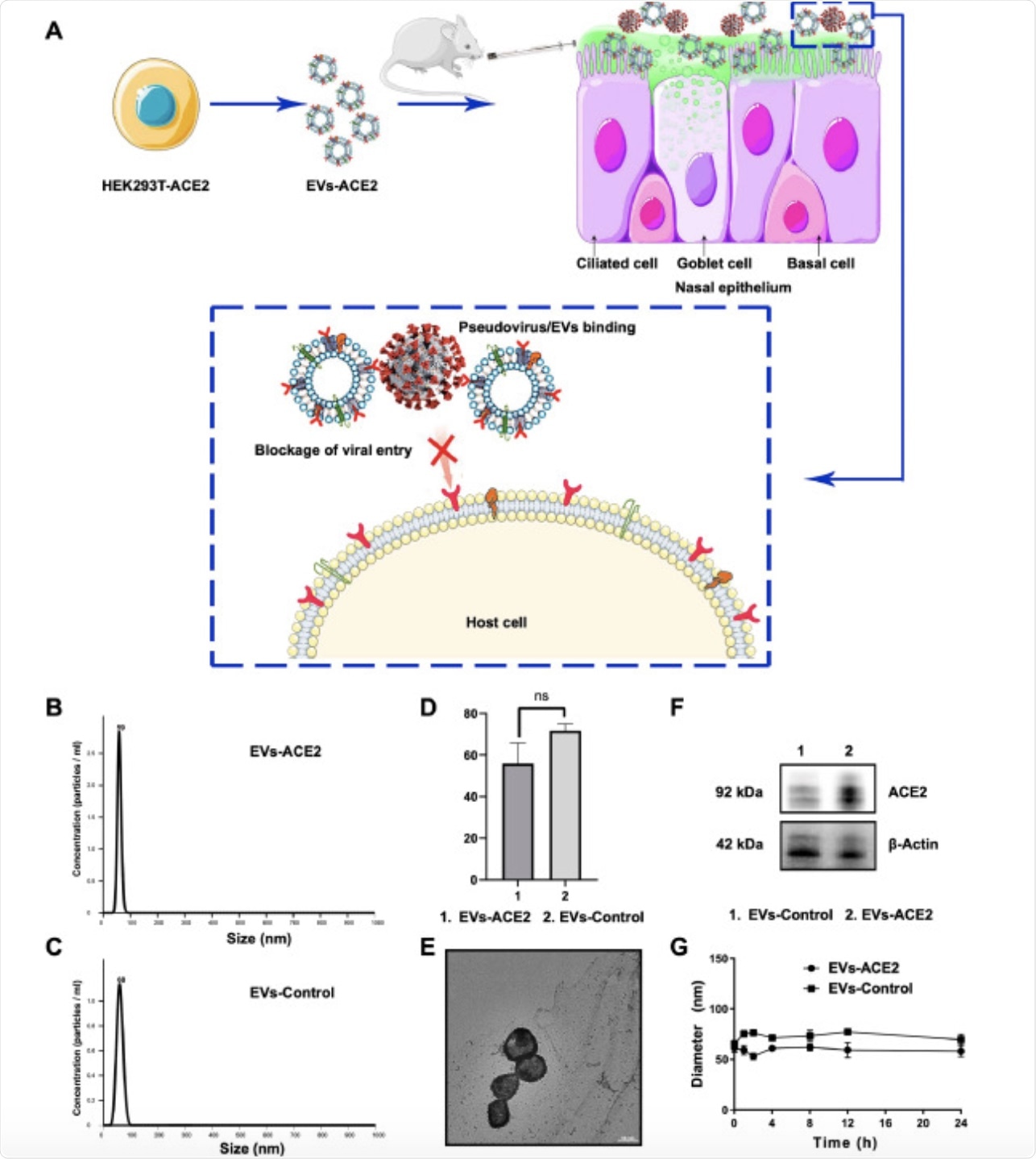 Figure 1. (A) Schematic mechanism of EVs-ACE2 inhibiting SARS-CoV-2 infection. The EVs-ACE2 were derived from the engineered HEK293T cells with stable ACE2 expression. EVs-ACE2 can competitively bind with the viruses via ACE2/S-protein interaction, thus blocking the virus to enter the host cells. (B) Size distributions of EVs-ACE2 measured by NTA. (C) Size distributions of EVs-control. (D) The median diameters of the EVs. (E) TEM images of EVs-ACE2. Scale bar = 50 nm. (F) ACE2 expression in EVs-ACE2 and EVs-Control. (G) The colloidal stability of the EVs. Data are presented as mean ± SD (n = 3); ns, no significance.
Figure 1. (A) Schematic mechanism of EVs-ACE2 inhibiting SARS-CoV-2 infection. The EVs-ACE2 were derived from the engineered HEK293T cells with stable ACE2 expression. EVs-ACE2 can competitively bind with the viruses via ACE2/S-protein interaction, thus blocking the virus to enter the host cells. (B) Size distributions of EVs-ACE2 measured by NTA. (C) Size distributions of EVs-control. (D) The median diameters of the EVs. (E) TEM images of EVs-ACE2. Scale bar = 50 nm. (F) ACE2 expression in EVs-ACE2 and EVs-Control. (G) The colloidal stability of the EVs. Data are presented as mean ± SD (n = 3); ns, no significance.
Study findings
The results of the in vitro studies found that EVs-ACE2 were quite effective in preventing the entry and infection of S-pseudovirus into host cells.
Previous research has found that higher viral loads are present in the nasal swabs of both symptomatic and asymptomatic patients as compared to throat swabs. The nasal epithelium cells contain ciliated cells and goblet cells that show higher ACE2 expression, thus indicating that it is a primary portal of entry of SARS-CoV-2 into the host cells.
The mice nasal epithelium also showed higher ACE2 expression. The results of this study indicated that the intranasal pre-administration of EVs-ACE2 was quite effective in capturing the S-pseudovirus and preventing its entry into the cells. The neutralization of the virus was significantly greater in mice receiving the EVs-ACE2 as compared to those that received the EVs-Control.
The current study also indicated that the S-pseudovirus positive rates in EVs-Control and blank control groups were 100 times higher as compared to the EVs-ACE2 group. Additionally, no major changes were observed in the blood chemistry test and histopathological analysis suggesting they could be safely used.
“As nasal mucosa is a front-line defense against respiratory infection in the human body, the intranasal administration of therapeutics could be promising in limiting the spread of COVID-19.”
Study takeaways
Physical protective equipment may not be able to provide complete protection in virus-rich indoor environments. Therefore, the development of intranasal medicine is of increased importance, as it could provide additional protection.x