The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide, precipitating the coronavirus disease 2019 (COVID-19) pandemic, with over 251 million confirmed infections and almost 5.1 million deaths reported over the last two years. Many papers have emerged since SARS-CoV-2 was originally identified that examine the immune response to the virus in order to help develop effective vaccines.
SARS-CoV-2 is a single-stranded positive-sense ribonucleic acid (RNA) virus that expresses four structural proteins, of which include the surface spike (S) glycoprotein, membrane protein (M), envelope protein (E), and nucleocapsid (N) protein. The S protein mediates virus-host cell receptor attachment and cell entry that occurs through its interaction with the angiotensin-converting enzyme 2 (ACE2) receptor.
Neutralizing antibodies produced against SARS-CoV-2 can inhibit viral entry, typically by blocking this interaction. The titer of these antibodies appears to be a correlate of robust protection against the infection following vaccination. The use of neutralizing monoclonal antibodies is also associated with the suppression of infection in animal experiments.
Most of these antibodies target the receptor-binding domain (RBD) of the S protein, though some bind to the N-terminal domain (NTD) of the S1 or fusion components within the S2 domain of the S protein as well.
B Cells and T-follicular helper cells in the antibody response
The antibody response is dependent on the activation of virus-specific B-cells. These cells, being primed by viral antigens, differentiate within the germinal center (GC) of the lymph nodes into long-lived, antibody-secreting plasma cells or memory B-cells (MBCs). Some of these cell products may be cross-reactive cells and are thus capable of recognizing epitopes that are common to SARS-CoV-2 and the endemic seasonal human coronaviruses.
Although these cells do not neutralize SARS-CoV-2, they may clear the free virus or eliminate infected cells. These effects are achieved by the Fc effector antibody functions such as antibody-dependent phagocytosis (ADP) and antibody-dependent cellular cytotoxicity (ADCC). Many monoclonal antibodies targeting the RBD and NTD act via these functions by binding to Fc cellular receptors.
Neutralizing antibodies are secreted by short-lived antibody-secreting cells (ASCs) that produce large antibody volumes during early infection. ASCs are detected between 7-14 days from symptom onset, reaching their peak titer at about 23 days until eventually declining. However, ASCs can remain detectable at up to 11 or more months from infection, with S protein-specific ASCs being found in the bone marrow over this period.
Fc-mediating anti-S protein antibodies are more durable and may protect against infection on subsequent exposures.
While neutralizing antibodies wane, S protein-specific MBCs increase in frequency in peripheral blood during convalescence. These cells can reach a plateau at about 8 months and remain stable for 12 or more months. Moreover, S protein-specific MBCs continue to evolve with new clones being produced between 6-12 months as more mutations appear in their genomes.
Convalescent sera-derived monoclonal antibodies show a varying affinity for the S or RBD, and higher neutralizing activity, depending on the time since symptom onset. The GC thus continues to be active with affinity maturation, resulting in the persistence of a class of MBCs that produce highly specific and effective antibodies over time.
Continuing antigen exposure may occur due to low-level viral replication in the gut, or from immune complexes within antigen-presenting cells.
Tfh cells in the antibody response
The T-follicular helper (Tfh) cells are one type of CD4 T-cells that are essential to activate B-cells. Additionally, Tfh cells promote their survival and differentiation into MBCs and long-lived plasma cells with high specificity and binding affinity through repeated interactions in the GC of B cell follicles.
Tfh cells express the chemokine receptor CXCR5 but lack CCR7. They also express CD45RO and Bcl-6, a transcriptional repressor. Tfh cells act on the B-cells that they recognize by CD40L and other costimulatory molecules, besides local cytokines like interleukin (IL)-21. The latter is correlated with humoral responses to the flu vaccine.
Tfh cells are mostly in lymphoid GCs; however, a small set circulates in the blood, which are known as circulating Tfh (cTfh). These cTfh cells express CXCR5, such as those in the lymphoid tissue, making up about 10% of the total CD4 cell population in the bloodstream.
There are differences between cTfh cells and lymphoid Tfh cells, including the lack of Bcl-6, and the absence of activation markers like PD-1 or CD38. These cells are thus more quiescent, despite their clear overlap with the GC Tfh cells. In fact, cTfh cells secrete higher amounts of IL-21 and IL-10 than CD4 T-cells without CXCR5 expression.
As a result, they are better equipped to promote B-cell survival and differentiation to ASCs. These cells, like conventional memory CD4 T-cells, express some T helper cell-associated chemokine receptors like CXCR3 and CCR6, as well as transcriptional factors defining the lineage such as T-bet, GATA3, ROR-γt. Upon activation, cTfh cells produce low levels of cytokines like interferon-gamma (IFNγ), IL-4, and IL-17.
How do Tfh cells work in SARS-CoV-2 antibody responses?
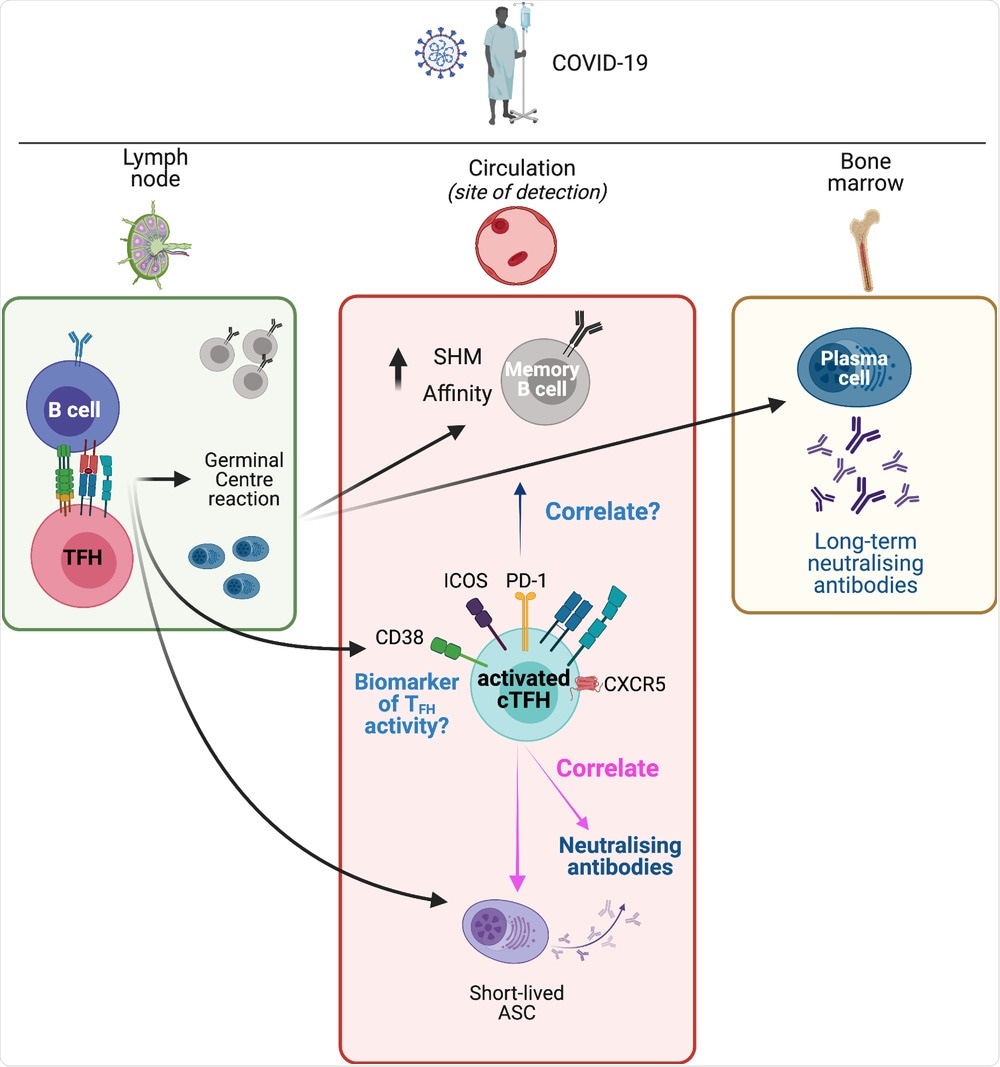 Lymphoid and circulating TFH responses in COVID-19. SARS-CoV-2 antigen in the lymph nodes results in activation of antigen-specific B cells and TFH cells. Their interaction leads to the initiation of the germinal center reaction. This results in the development of memory B cells with increased somatic hypermutation (SHM) and increased affinity, as well as long-lived plasma cells that traffic to the bone marrow and provide a long-term source of neutralizing antibodies. A population of short-lived antibody-secreting cells (ASCs) appears in the circulation and provides a raid source of neutralizing antibodies. Concurrently, a population of activated (CD38+, PD-1+, ICOS+) cTFH cells appears in the circulation. This population contains antigen-specific cTFH cells (not depicted). Although memory B cells and ASCs are primarily located in lymphoid tissues, they are typically measured in blood samples, where they correlate with activated cTFH cells. Activated cTFH cells also correlate with the development of neutralizing antibodies. These cTFH cells are a potential biomarker of TFH activity in lymphoid tissues but it remains to be determined if this population of cTFH cells are predictive of long-term neutralizing antibodies, or of the development of long-lived plasma cells and the prolonged evolution of the MBC pool. The figure was created with BioRender.com.
Lymphoid and circulating TFH responses in COVID-19. SARS-CoV-2 antigen in the lymph nodes results in activation of antigen-specific B cells and TFH cells. Their interaction leads to the initiation of the germinal center reaction. This results in the development of memory B cells with increased somatic hypermutation (SHM) and increased affinity, as well as long-lived plasma cells that traffic to the bone marrow and provide a long-term source of neutralizing antibodies. A population of short-lived antibody-secreting cells (ASCs) appears in the circulation and provides a raid source of neutralizing antibodies. Concurrently, a population of activated (CD38+, PD-1+, ICOS+) cTFH cells appears in the circulation. This population contains antigen-specific cTFH cells (not depicted). Although memory B cells and ASCs are primarily located in lymphoid tissues, they are typically measured in blood samples, where they correlate with activated cTFH cells. Activated cTFH cells also correlate with the development of neutralizing antibodies. These cTFH cells are a potential biomarker of TFH activity in lymphoid tissues but it remains to be determined if this population of cTFH cells are predictive of long-term neutralizing antibodies, or of the development of long-lived plasma cells and the prolonged evolution of the MBC pool. The figure was created with BioRender.com.
In an individual who has been infected with or vaccinated against SARS-CoV-2, a subset of activated cTfh cells appear in the blood for a short duration. This contains CD4 T-cells that react to antigens in the vaccine or virus. This subset is linked to the appearance of ASCs and the antibody response in quantitative and qualitative terms.
The activation of cTfh cells is thus a robust marker of normal healthy antibody responses to viral antigens. However, different cTfh subsets have different associations with antibody production.
This has been observed after the administration of influenza, human papillomavirus, (HPV) malaria, Ebola, and hepatitis B vaccines, as well as many other vaccines, and also following acute infection with several of these pathogens. Thus, cTfh cells can be used to understand how humoral immunity works.
In COVID-19, one subset of cTfh cells is activated with reduced CCR7 expression and PD-1+ICOS+ expression and remains active for about two weeks from symptom onset. The emergence of S protein-specific cTfh cells during acute infection is followed by their persistence for six or more months.
At this late point, there are fewer CXCR3–CCR6+ S-specific cTFH cells than in either the acute stage or early convalescence. Yet, COVID-19 patients who have recovered demonstrate IFNγ and IL-21 production from antigen-specific cTfh cells.
The cTfh activation in acute COVID-19 is a measure of anti-RBD IgM antibody titers and avidity. Similarly, CXCR3+ cTfh1 cells are associated with high anti-S protein neutralizing antibodies. Thus, the phenotypic polarization of cTfh cells is a key feature that characterizes the robust development of an antibody response.
“Altogether, the current data indicate that CXCR3+ cTFH1 cells are a strong correlate of neutralizing and total antibodies against SARS-CoV-2, while the role of CXCR3– cTFH2 and cTFH17 cells requires further investigation.”
In fact, cTfh cell frequencies that react with the SARS-CoV-2 S, N, and M antigens are associated with neutralizing titers. In certain cases, severe COVID-19 is reported to be associated with reduced Tfh cell frequencies, with higher frequencies of cytotoxic cTfh cells expressing granzyme B and perforin.
Further research is needed in this area because of the expectation that poor Tfh activity would cause lower antibody titers.
Vaccination with the current messenger ribonucleic acid (mRNA) vaccines induces robust GC reactions, with spike-specific cTfh cell activation. These produce IFNγ but not IL-17, peaking at one month and then waning. Therefore, the cells produced following vaccination are unlike the anti-S protein Th1 cells that remain stable for six months or more.
Implications
The current analysis indicates that cTfh cells react to the S antigen following vaccination are associated with anti-S protein neutralizing antibodies, as well as to SARS-CoV-2 variants of concern. These cells also correlate with S and RBD specificity in the MBC response at one month from vaccination, thus indicating that cTfh cells are markers for these immunological events.
Vaccination following natural infection and recovery is always linked with better anti-S protein cTfh cell responses as compared to vaccinated naïve individuals. The frequency of these cells before vaccination is proportional to the post-vaccination neutralizing titers against both ancestral and VOC strains of SARS-CoV-2.
It is clear that cTfh cells are important biomarkers of antibody responses after vaccination. Questions that remain include:
- Why do only some cTfh subsets associate with antibody titers?
- Are cTfh cells markers of long-term neutralizing activity?
- Do cTfh cells predict the presence of long-lived plasma cells?
With a better understanding of how long the GC Tfh responses last and how they are linked to cTfh phenotype and frequency, these cells can be used as the basis of a more effective vaccination strategy.